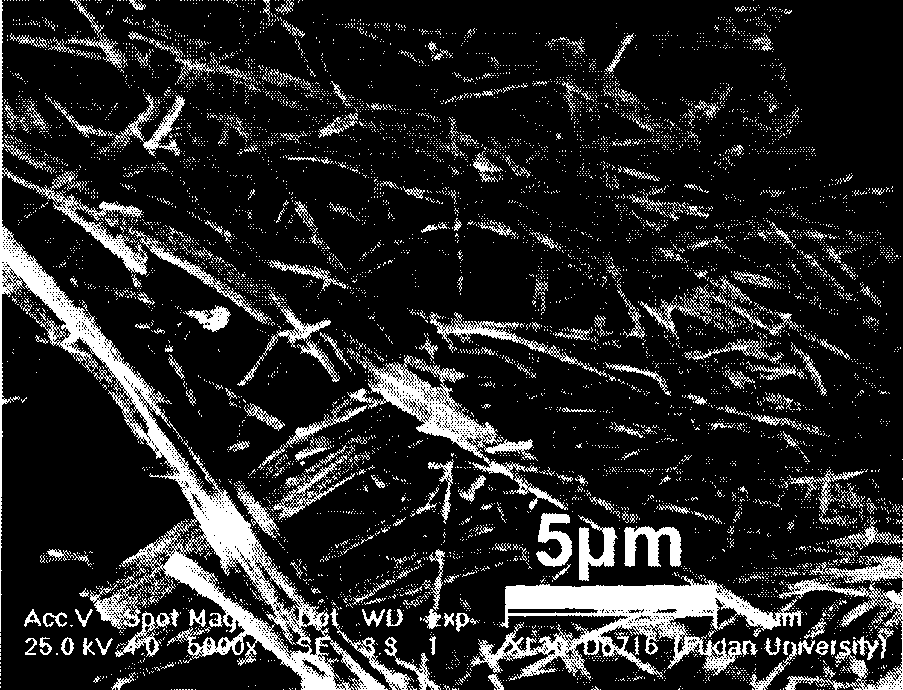
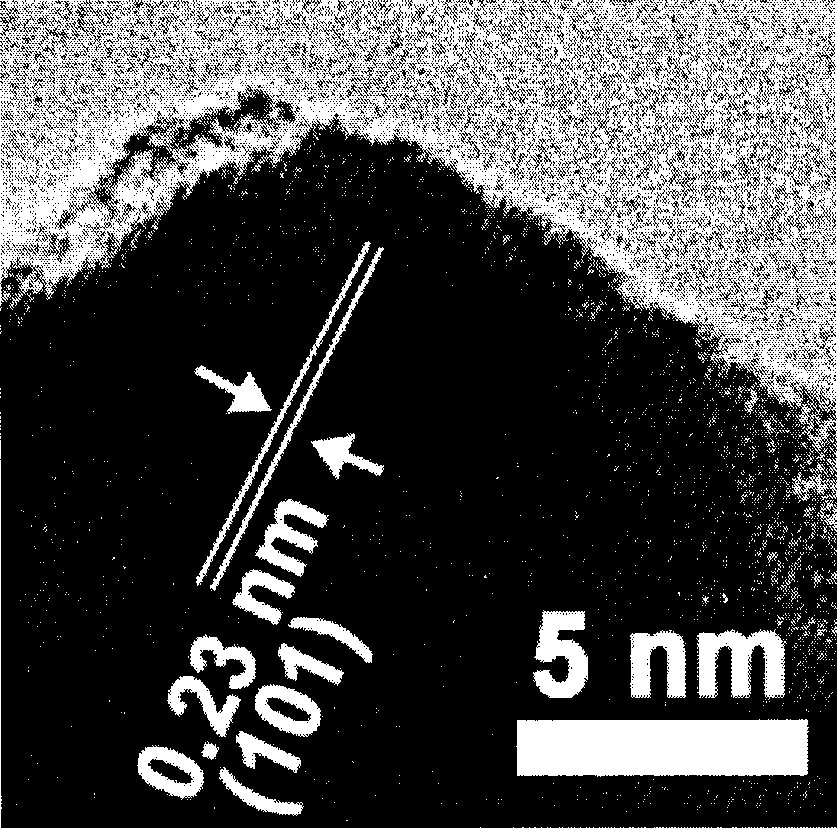
Synthesis of stephanoporate molybdenum carbide nano-wire
A synthesis method and molybdenum carbide technology, applied in the field of nanomaterials, can solve the problems of high cost, cannot be completely eliminated, and high atmosphere requirements, achieve product quality and high yield, good application and industrialization prospects, and simple and easy-to-control preparation conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A method for synthesizing porous molybdenum carbide nanowires, characterized in that it comprises the following steps:
[0048] (1) 1.24g ammonium molybdate (NH 4 ) 6 Mo 7 o 24 4H 2 O is dissolved in 20ml distilled water to obtain ammonium molybdate solution (the molar concentration of molybdenum atom is about 0.35mol / L);
[0049] (2) Inject 1.90g of aniline into the ammonium molybdate solution (the molar ratio of aniline to molybdenum atoms is about 2.92:1);
[0050] (3) Add 1.0 mol / L hydrochloric acid dropwise to the solution to adjust the pH to 4-5 until white precipitates appear to obtain a reaction solution;
[0051] (4) The reaction solution was moved to an oil bath at 50°C, and reacted for 6 hours to obtain the product;
[0052] (5) The product was washed several times with absolute ethanol, filtered with suction, and dried at 50°C;
[0053] (6) Finally, the product was calcined in an argon flow at a calcining temperature of 725° C. for a calcining time of...
Embodiment 2
[0060] A method for synthesizing porous molybdenum carbide nanowires, characterized in that it comprises the following steps:
[0061] (1) ammonium molybdate (NH 4 ) 6 Mo 7 o 24 4H 2 O is dissolved in 20ml distilled water to obtain ammonium molybdate solution (the molar concentration of molybdenum atom is 0.02mol / L);
[0062] (2) Inject aniline into the ammonium molybdate solution (the molar ratio of aniline to molybdenum atoms is 4.0-2.0:1);
[0063] (3) Add 1.0 mol / L hydrochloric acid dropwise to the solution to adjust the pH to 4-5 until white precipitates appear to obtain a reaction solution;
[0064] (4) The reaction solution was moved to an oil bath at 50°C, and reacted for 6 hours to obtain the product;
[0065] (5) The product was washed several times with absolute ethanol, filtered with suction, and dried at 50°C;
[0066] (6) Finally, the product was calcined in an argon flow at a calcining temperature of 725° C. for a calcining time of 5 hours to obtain a por...
Embodiment 3
[0068] A method for synthesizing porous molybdenum carbide nanowires, characterized in that it comprises the following steps:
[0069] (1) ammonium molybdate (NH 4 ) 6 Mo 7 o 24 4H 2 O is dissolved in 20ml distilled water to obtain ammonium molybdate solution (the molar concentration of molybdenum atom is 1.5mol / L);
[0070] (2) Inject aniline into the ammonium molybdate solution (the molar ratio of aniline to molybdenum atoms is 4.0-2.0:1);
[0071] (3) Add 1.0 mol / L hydrochloric acid dropwise to the solution to adjust the pH to 4-5 until white precipitates appear to obtain a reaction solution;
[0072] (4) The reaction solution was moved to an oil bath at 50°C, and reacted for 6 hours to obtain the product;
[0073] (5) The product was washed several times with absolute ethanol, filtered with suction, and dried at 50°C;
[0074] (6) Finally, the product was calcined in an argon flow at a calcining temperature of 725°C for a calcining time of 5 hours to obtain a porous...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com