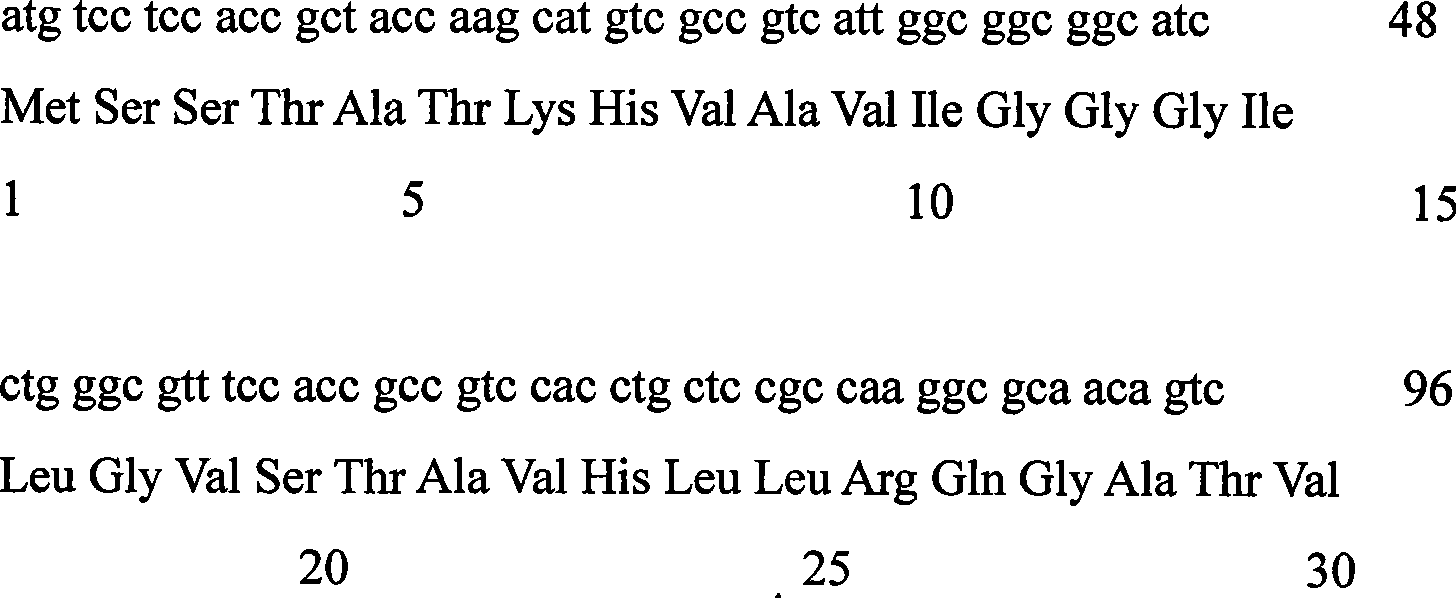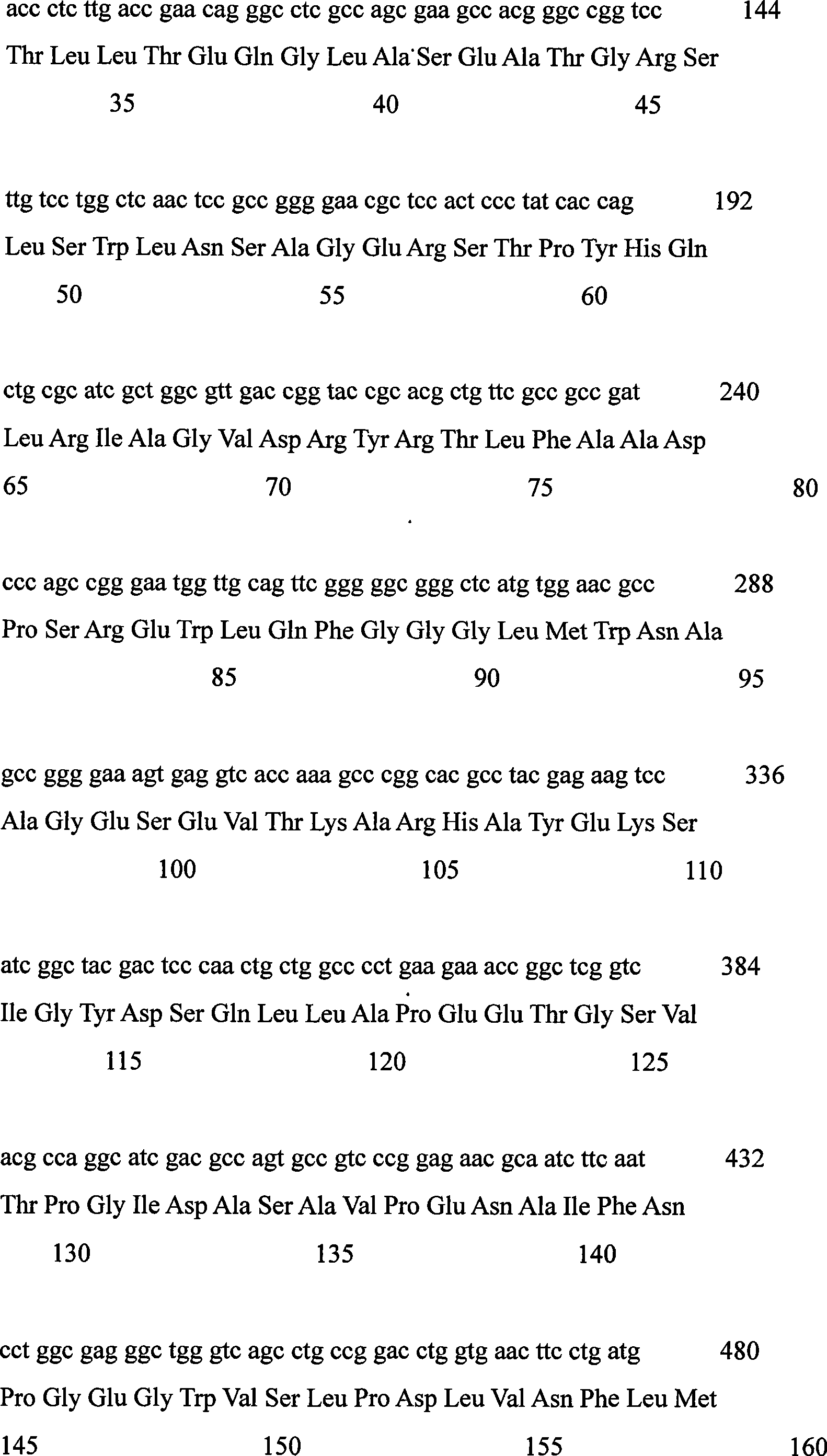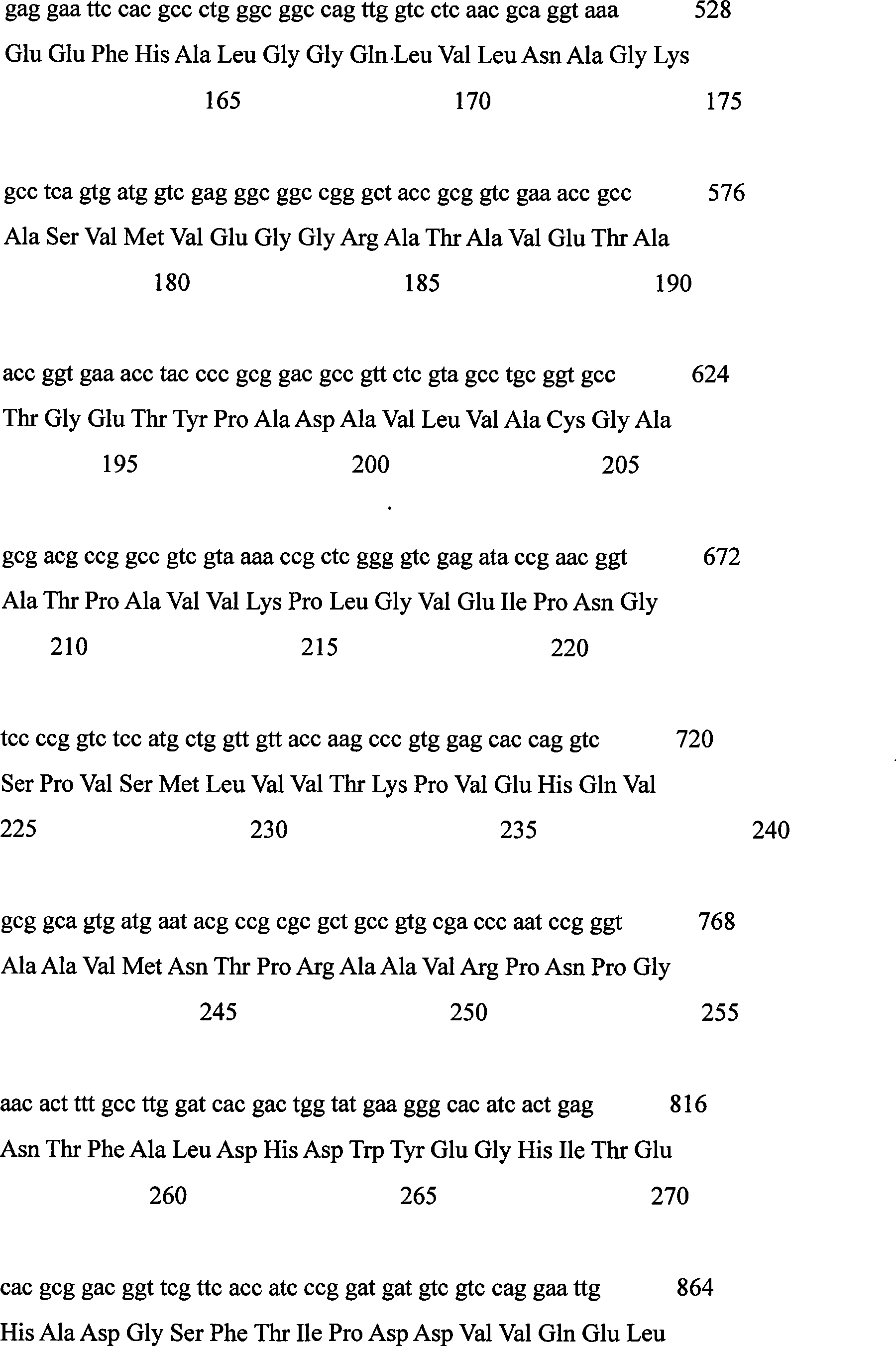Levulose valine oxidizing enzyme of high activity and method of producing the same
A valine oxidase and high-activity technology, which is applied in the field of preparation of high-activity fructose valine oxidase, can solve the problems of long time and high demand for enzyme amount, and achieve the goals of reduced enzyme amount, high activity, and shortened reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The FVO gene coding sequence of Corynebacterium sp.2-4-1 was used as a template for error-prone PCR amplification.
[0022] 1. Primer sequence:
[0023] Forward primer: 5’-TTGTTCGGATCCATGTCCTCCACCGCTAC-3’
[0024] Reverse primer: 5’-TTGTTCAAGCTTCTAGGAGAACCGGCCCG-3’
[0025] 2. Error-prone PCR reaction system and reaction conditions:
[0026] PCR reaction system:
[0027] The 100μL system contains:
[0028] 10mM Tris-HCl pH 8.30, 50mM KCl, 6.5mM MgCl 2 ,
[0029] 0.15mM MnCl 2 , 0.2mM dGTP / dATP, 0.8mM dTTP / dCTP,
[0030] 2.2μg SSB Protein, 0.5μM forward / reverse primer, 10ng template DNA
[0031] 2.5 units of TaqDNA polymerase.
[0032] PCR reaction conditions:
[0033] 94℃ 5min; 94℃ 30sec, 55℃ 30sec, 72℃ 2min, 35 cycles; 72℃ 10min; 4℃forever.
[0034] 3. Take 3μL of the error-prone PCR product on a 1% agarose gel electrophoresis, and a product band of about 1kb can be seen. The error-prone PCR product was purified and recovered with a purification and recovery kit, connected...
Embodiment 2
[0036] Initial screening of mutant strains:
[0037] The plasmid DNA in the mutant library was extracted and digested with BamH I and Hind III to recover a target fragment of about 1 kb. The vector pGEX-4T-1m was also digested with BamH I and Hind III and recovered, and the two were connected at 4°C. Overnight, transform into BL-21 strain and spread on LB containing IPTG, fructose valine and N-(carboxymethylaminocarbonyl)-4,4-bis(methylamino)-benzidine [DA-64] Plate: After culturing at 37°C for 12-24 hours, obvious discoloration can be seen. The clones with fast discoloration and large discoloration circles were picked and cultured in liquid medium. A total of 48 clones were picked.
Embodiment 3
[0039] Quinone method detection of mutant enzyme activity:
[0040] 1. After 4 hours of inducing expression, collect the bacterial liquid, measure and record the OD600 value of the cell culture.
[0041] 2. Prepare bacterial lysate for detecting enzyme activity. In 374 μL B-PER TM Resuspend the cells in bacterial protein extraction reagent (Pierce product78248), then add 50uL protease and phosphorylase inhibitors and bacterial cell extract (Sigma product P8465), 1uL 34mg / mL chloramphenicol (prepared with methanol). Vortex quickly for one minute. Place on ice for 5 minutes. Centrifuge for 1 min and place on ice.
[0042] 3. Use the Bradford method to determine the protein content.
[0043] 4. Take 50μL of the above bacterial lysate and add it to the quinone method reaction mixture, incubate at 37°C for 1-3min, and measure the absorbance at 555nm. The components of the reaction mixture are: 100mM potassium phosphate buffer (pH8.0), 1purpurogallin unit / ml peroxidase, 0.45mM 4-aminoan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com