Method for preparing palladium catalyst with alkali resistance for production of hydrogen peroxide and product
A hydrogen peroxide and palladium catalyst technology, which is applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of catalyst strength decline, catalyst service life decline, activity Eliminates problems such as component palladium falling off, and achieves the effect of improving industrial applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Alumina powder is prepared by neutralizing aluminum sulfate, sodium aluminate and ammonia water, and extruded into Clover strips were baked in an oven at 120°C for 10 hours and roasted at 800°C for 6 hours to obtain an alumina carrier. 500 grams of alumina carrier was impregnated with a mixed solution of 32.5% magnesium nitrate and manganese nitrate with a weight of 715g and a concentration of 32.5%. After washing with water, bake in an oven at 120°C for 10 hours, bake at 900°C for 4 hours, then impregnate with 43.6g of sodium nitrate with a concentration of 40% for 1 hour, and bake in an oven at 120°C for 8 hours to obtain a composite carrier.
[0046] With the ratio of palladium chloride and composite carrier as 0.53%, get 300g composite carrier equal volume impregnation containing 1.59g PdCl 2 Immerse in the solution for 0.5 hours at 80°C, drain and wash, bake at 120°C for 8 hours, and bake at 500°C for 4 hours to obtain sample 1.
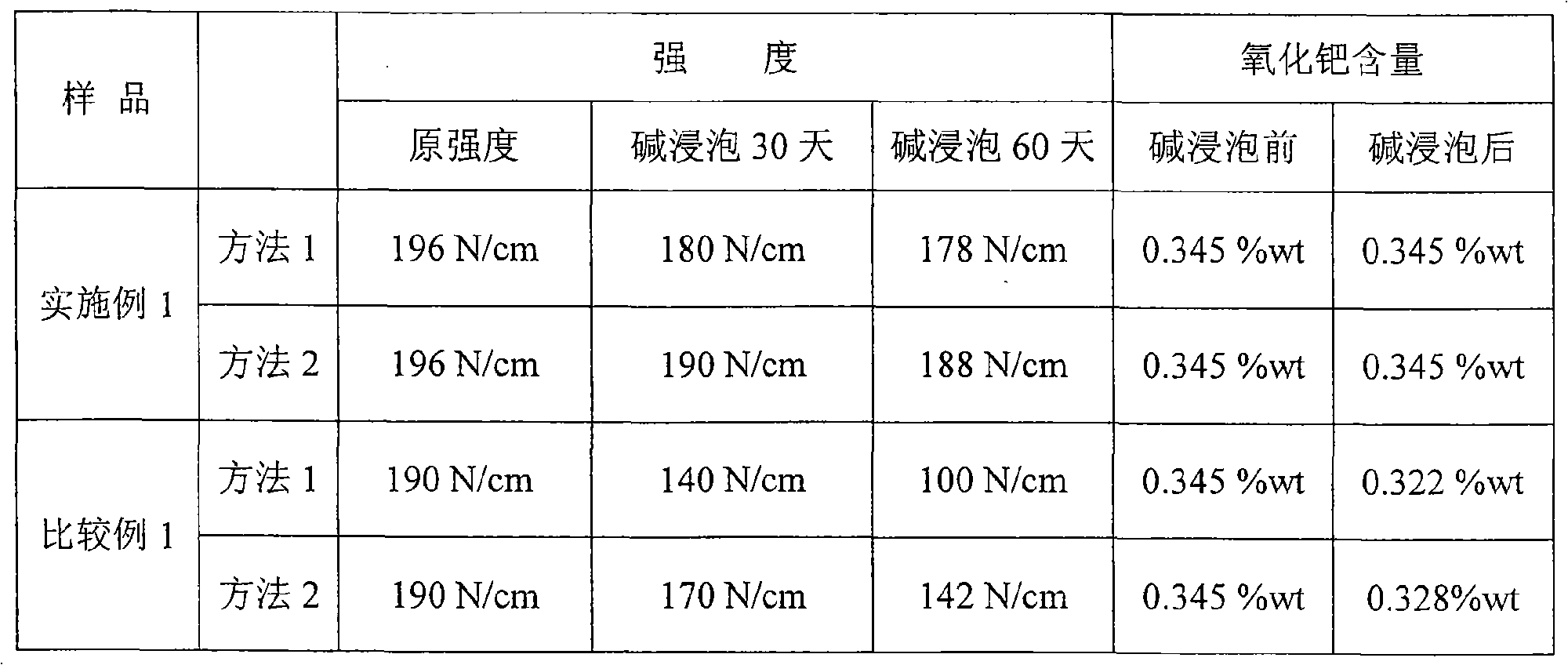
[0047] The strength of sample 1 ...
Embodiment 2
[0051] Dry rubber powder in embodiment 1 is made by roll forming method Spherical type, the rest of the conditions remain unchanged, and sample 2 is prepared.
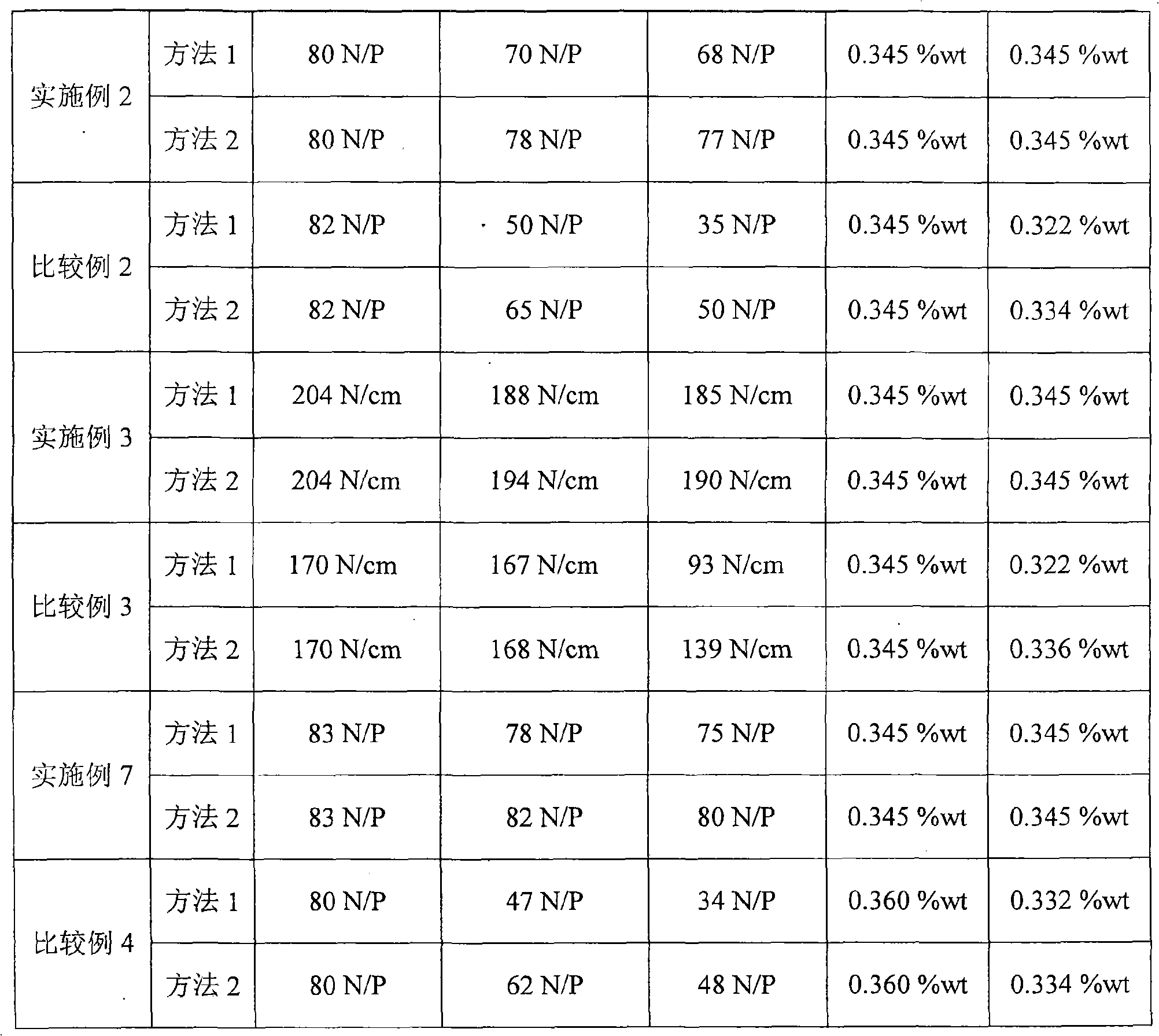
[0052]The strength of sample 2 is 80N / P, the palladium oxide content is 0.345%wt, the hydrogen efficiency is 5.6g / L, and the activity is 3.76KgH 2 o 2 (100%) Kg Cat·d.
[0053] Method 1: After soaking for 30 days, the strength is 70N / P, after soaking for 60 days, the strength is 68N / P, and the content of palladium oxide is 0.345%wt;
[0054] Method 2: After soaking for 30 days, the strength is 78N / P, after soaking for 60 days, the strength is 77N / P, and the content of palladium oxide is 0.345%wt.
Embodiment 3
[0056] In Example 1, the solution of magnesium nitrate and manganese nitrate was changed to 953g, the concentration was 32.5% mixed solution of magnesium nitrate and cerium nitrate, and the other conditions were unchanged, and sample 3 was obtained.
[0057] The strength of sample 3 is 204N / cm, the palladium oxide content is 0.345%wt, the hydrogen efficiency is 6.8g / L, and the activity is 4.57Kg H 2 o 2 (100%) Kg Cat·d.
[0058] Method 1: After soaking for 30 days, the strength is 188N / cm, after soaking for 60 days, the strength is 185N / cm, and the content of palladium oxide is 0.345%wt;
[0059] Method 2: After soaking for 30 days, the strength is 194N / cm, and after soaking for 60 days, the strength is 190N / cm, and the content of palladium oxide is 0.345%wt.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com