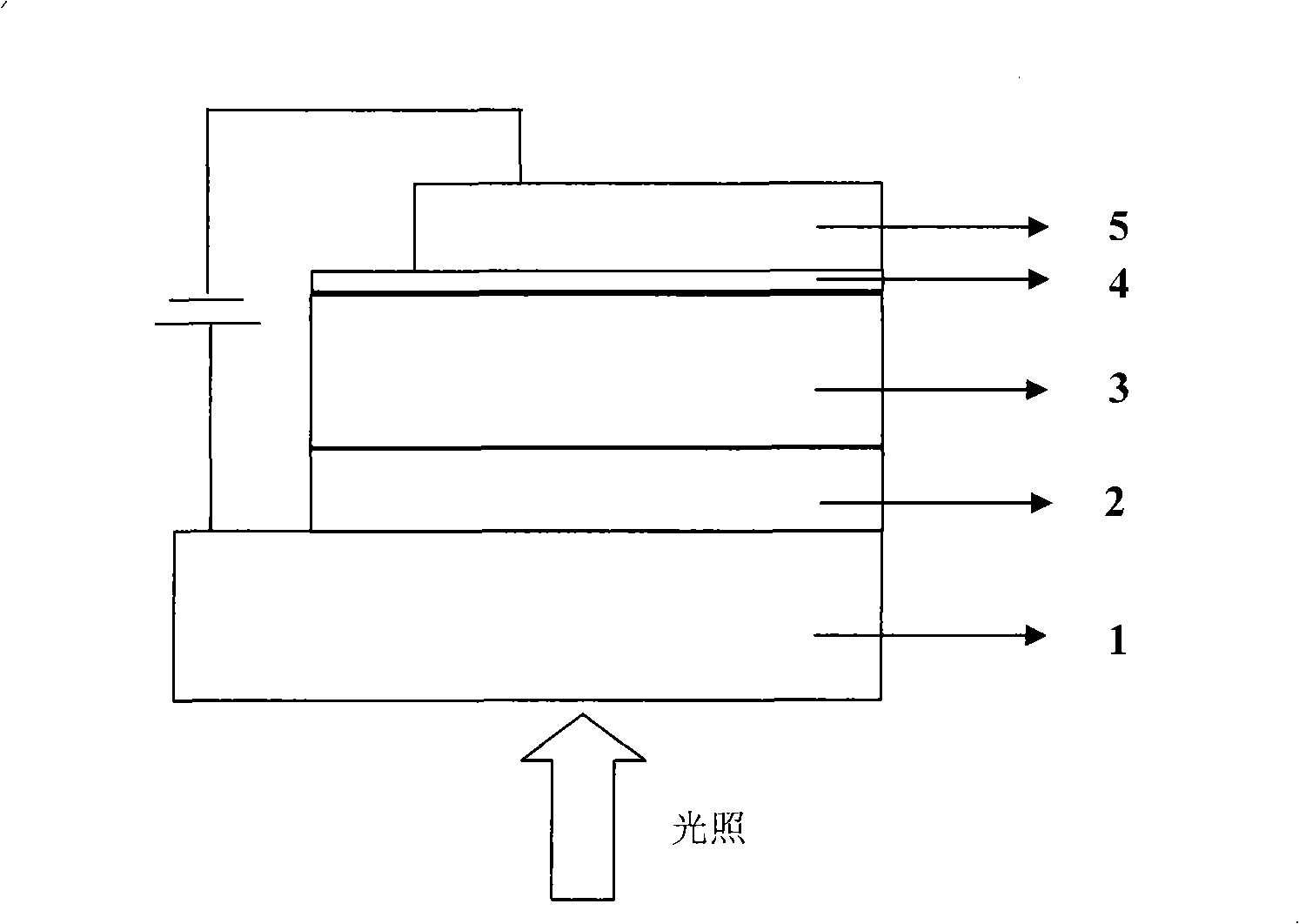
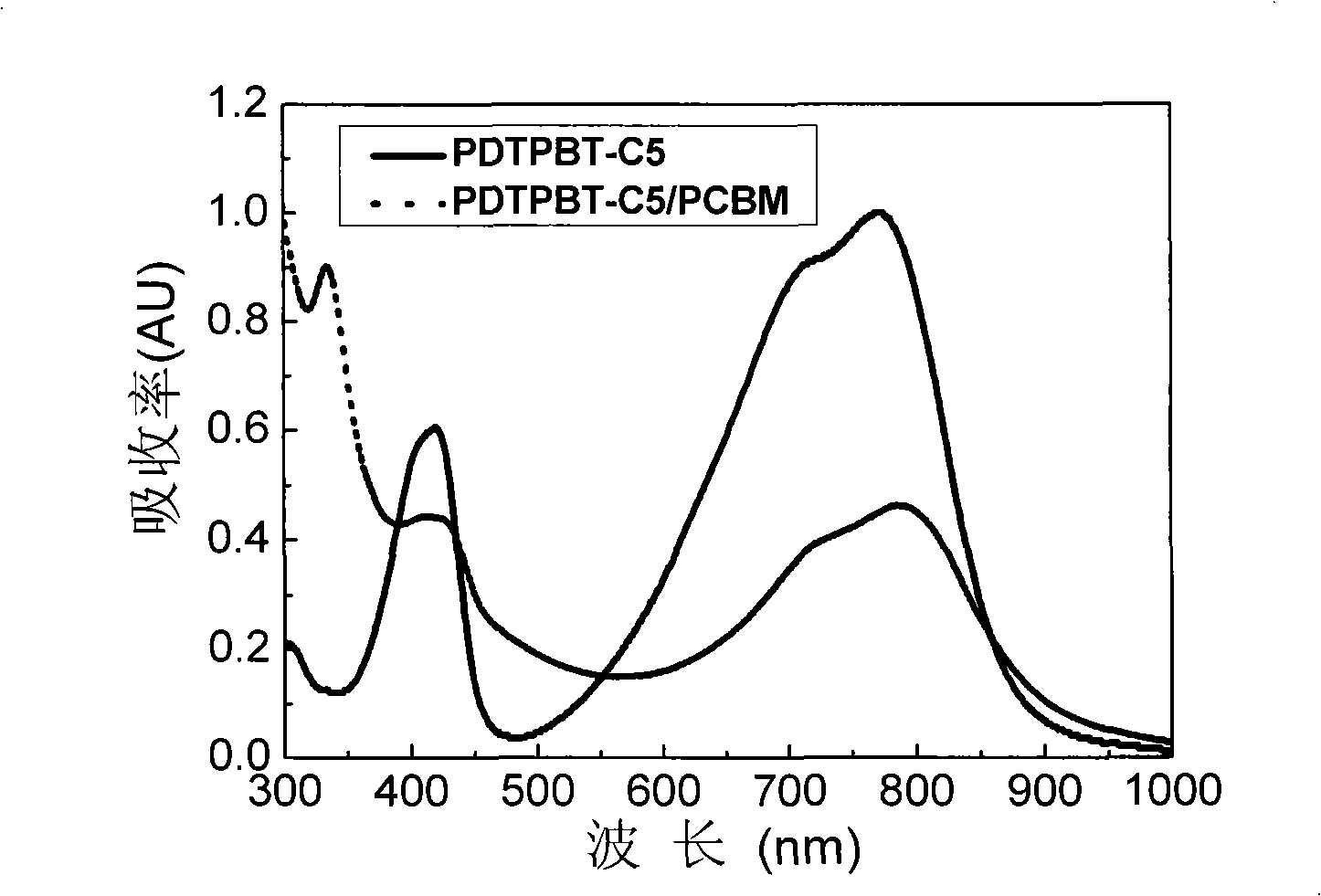
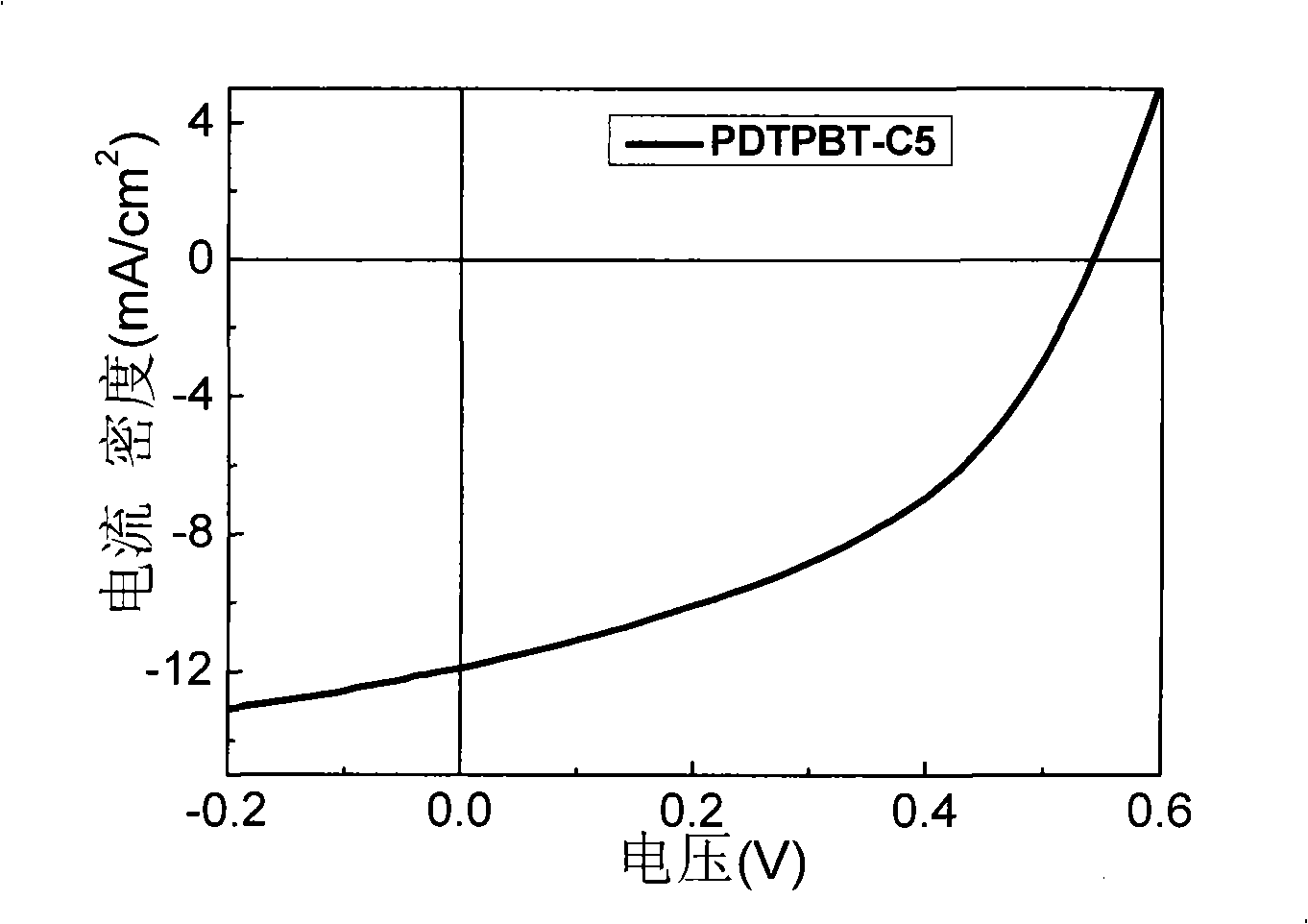
Donor-receptor type conjugated polymer containing dithiophen b pyrrole, preparation method and application thereof
A conjugated polymer, dithiophene technology, applied in semiconductor/solid-state device manufacturing, electrical solid-state devices, semiconductor devices, etc., can solve the problems of limited types of narrow-bandgap polymers, limited large-scale applications, and difficulty in the synthesis of PCPDTBT. The effect of high energy conversion efficiency, good planarity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of N-substituted dithiophene[3,2-b:2',3'-d]pyrrole bismethyl tin salt monomer
[0039]
[0040] Referring to the method disclosed in Macromolecules., 2007, 40, 4173-4181, using 3,3'-dibromo-4,4'-disubstituted dithiophene and primary amine as raw materials, the compound containing Dithiopheno[3,2-b:2',3'-d]pyrroles with different substituents. Dithiophene[3,2-b:2',3'-d]pyrroles with different substituents were converted to dithiophene[3,2- b: 2',3'-d]pyrrole bismethyl tin salt monomer, yield 43%-92%.
[0041] Take the preparation of 2,6-ditrimethyltin-N-(1-pentylhexyl)dithiophene[3,2-b:2',3'-d]pyrrole as an example to illustrate in detail below, the synthesis steps are as follows :
[0042]
[0043] (1) N-(1-pentylhexyl)dithiophene[3,2-b:2',3'-d]pyrrole
[0044] 3,3'-Dibromobithiophene (2.43g, 7.50mmol), sodium tert-butoxide (1.73g, 18.0mmol), tris(dibenzylideneacetone)dipalladium (Pd 2 dba 3 , 0.172g, 0.190mmol) and 2,2'-bis(diphenylph...
Embodiment 2
[0047] Example 2: Preparation of 4,7-bisthienyl-2,1,3-benzothiadiazole
[0048]
[0049] Referring to the method disclosed in J.Mater.Chem., 2002, 12, 2887-2892, using 2-bromo-3,4-disubstituted thiophene as a raw material, debromination with butyllithium at -78°C and then adding Tributyltin chloride to obtain thiophene monotin salt, react it with 4,7-dibromobenzothiadiazole through Stille reaction to obtain bisthienyl-substituted benzothiadiazole, and then use NBS for bromination to obtain Dibromo-substituted Ar acceptor unit, yield 30-35%.
[0050] Below take the preparation of 4,7-bis(5-bromo-2-thiophene)-2,1,3-benzothiadiazole as an example to be specifically described, and the synthesis steps are as follows:
[0051]
[0052] (1) Tributyl-2-thienstannane
[0053] Add 2-bromothiophene (6.11 g, 37.5 mmol) into the reaction flask, and then add 100 mL of tetrahydrofuran. Cool down to -78°C, add n-butyllithium solution (16.5 mL, 41.2 mmol, 2.5M hexane solution) dropwis...
Embodiment 3
[0058] Example 3: Preparation of 2,3-disubstituted-5,7-dibromothieno[3,4-b]pyrazine
[0059]
[0060] Referring to the method disclosed in J.Org.Chem., 2002, 67, 9073-9076, using 2,5-dibromo-3,4-dinitrothiophene as a raw material, first reduce it to 3 with tin powder , 4-diaminothiophene, and then react with substituted glyoxal to obtain 2,3-disubstituted thieno[3,4-b]pyrazine, and finally use NBS bromination to obtain 2,3-disubstituted-5, 7-dibromothieno[3,4-b]pyrazine monomer, the yield is 70-90%.
[0061] Below take the preparation of 5,7-dibromothieno[3,4-b]pyrazine as an example to be specifically described, and the synthesis steps are as follows:
[0062]
[0063] (1) 3,4-diaminothiophene
[0064] Add 2,5-dibromo-3,4-dinitrothiophene (3.00g, 9.04mmol) and 54mL concentrated hydrochloric acid into the reaction flask, add ice bath to cool, slowly add tin powder (7.48g, 63.0mmol) to make the reaction The temperature of the material is maintained at 25-30°C, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Short circuit current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com