Conjugate, and preparation and use thereof
A conjugate and conjugation technology, applied in the field of conjugates and their preparation, can solve the problems of controversies in the treatment effect of patients, easy drug resistance and other problems, and achieve prolonged survival, good tolerance, and less toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
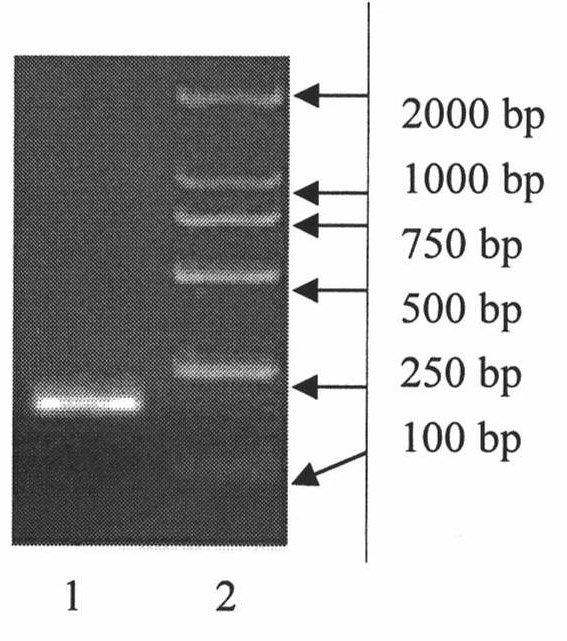
[0065] Embodiment 1, the preparation of recombinant human epidermal growth factor mutant mixture (rhEGF)
[0066] 1. Cloning of recombinant human epidermal growth factor coding gene
[0067] According to the codon bias of yeast, the full-length DNA sequence encoding mature recombinant human epidermal growth factor was artificially synthesized, and the gene was named as its nucleotide sequence as shown in sequence 1 in the sequence listing, and the encoded amino acid sequence as shown in the sequence listing Shown in Sequence 2.
[0068] Using the full-length DNA sequence of the above-mentioned synthetic recombinant human epidermal growth factor as a template, use Primer 5.0 software to design primers: upstream primer: 5-CCG CTCGAG AACTCAGATAGTGAATGCC-3, downstream primer: 5-CCG GAATTC TCAACGTAATTCCCACCA-3, in which the underlined parts are the restriction sites of XhoI and EcoRI, respectively, were amplified by PCR. The PCR reaction system is: 0.5 μl template, 5 μl 10×PC...
Embodiment 2
[0076] Embodiment 2, the preparation of Neisseria meningitidis outer membrane protein (P64k)
[0077] 1. Cloning of the outer membrane protein coding gene of Neisseria meningitidis
[0078] According to the sequence of Neisseria meningitidis of GenBank Accession No.X77920.1, use Primer 5.0 software to design primers: upstream primer: 5-CATG CCATGG CTTTAGTTGAATTGAA-3, downstream primer: 5-CCG GAATTCTTATTTTTTCTTTTGCGGAG-3, where the underlined parts are the restriction sites of NcoI and EcoRI respectively. Neisseria meningitidis CMCC 29336 (purchased from China Medical Bacteria Collection Center, referred to as CMCC) was boiled at 100°C for 10 min, and 3 μl was taken as a template for PCR amplification. The PCR reaction system is: 3 μl template, 5 μl 10×PCR buffer, 1 μl 10 mmol / L dNTP, 1 μl Pyrobest high-fidelity DNA polymerase (Shanghai Sangong product), 0.5 μl each of upstream and downstream primers with a final concentration of 0.5 μmol / L, Add ultrapure water to 50 μl. ...
Embodiment 3
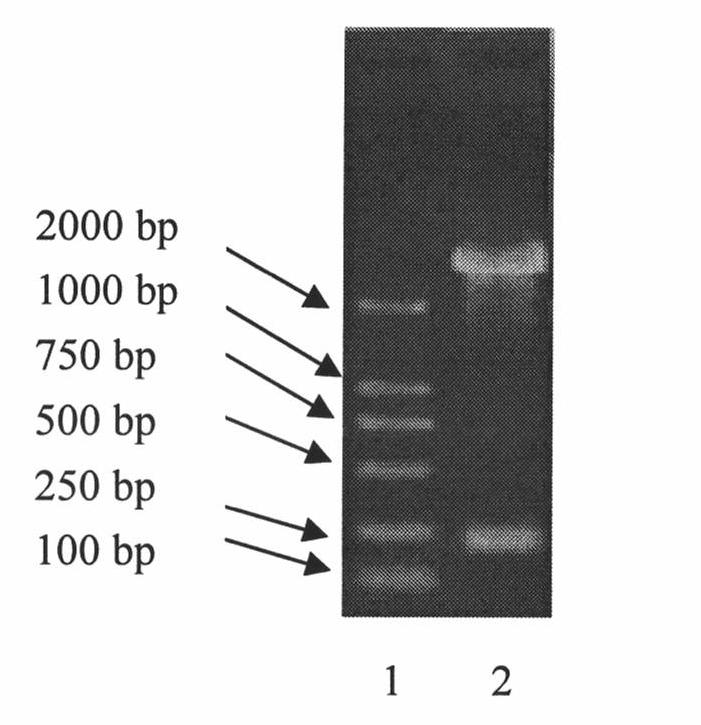
[0085] Example 3, Preparation of recombinant human epidermal growth factor mutant mixture and Neisseria meningitidis outer membrane protein conjugate (rhEGF-P64k)
[0086] Use 0.1M sodium borate solution (pH8.5) to adjust the concentration of the mixture of the recombinant human epidermal growth factor mutant prepared in the above example 1 and the P64k prepared in the above example 2 to 1.0 mg / mL respectively, and take different volumes of Concentration is the mixture of the recombinant human epidermal growth factor mutant of 1.0mg / mL and P64k protein solution in the aseptic pyrogen-free reaction kettle of 10L, the mol ratio that makes the mixture of recombinant human epidermal growth factor mutant and P64k is ( 12-8):1. Mix the above mixed solution in the reaction kettle and equilibrate to room temperature, slowly add 0.5% glutaraldehyde solution by volume, so that the final concentrations of glutaraldehyde in the mixed solution are 0.01%, 0.05% and 0.1% respectively . Aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com