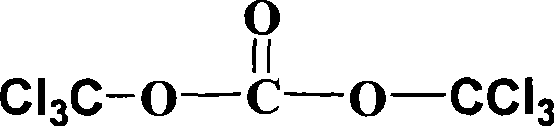Method for preparing water soluble pegylation hydroxycamptothecine derivatives
A technology of PEGylated hydroxycamptothecin and hydroxycamptothecin, applied to the preparation of water-soluble PEGylated hydroxycamptothecin derivatives, solid phosgene to prepare water-soluble PEGylated hydroxycamptothecin derivatives In the field of pharmaceuticals, it can solve the problems of rare PEGylation of hydroxycamptothecin, and achieve the effects of simple synthesis method, convenient storage, and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
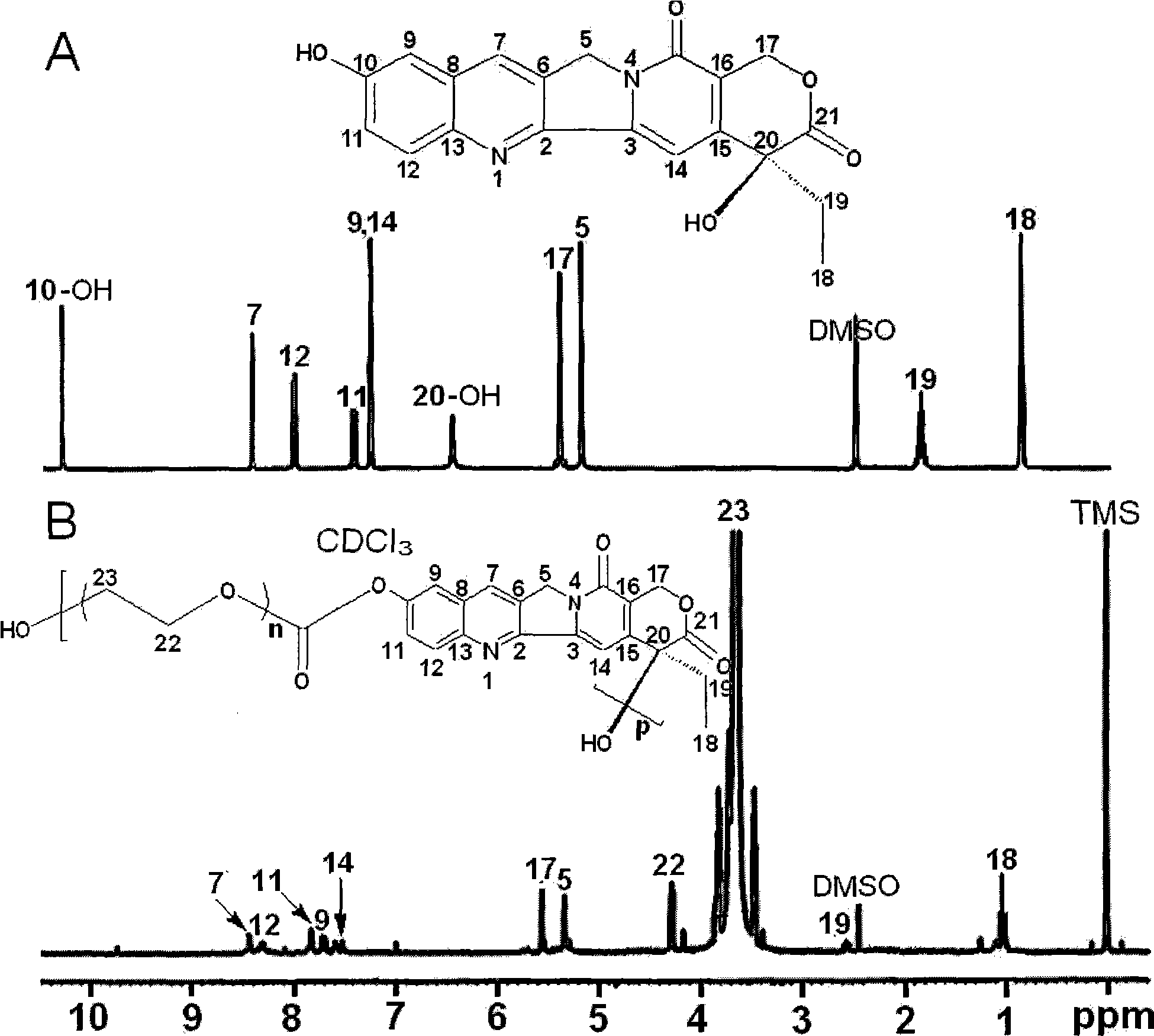
[0049] Embodiment 1. Preparation of water-soluble polyethylene glycol (PEG)-based hydroxycamptothecin (HCPT) derivatives
[0050] (1) Take by weighing 6.00g polyethylene glycol (PEG) 6000 and 0.36g hydroxycamptothecin (HCPT) and join in the round bottom flask that condensation tube, thermometer, constant pressure dropping funnel and magnetic stirring are housed, and measure Take 35mL of chloroform and pour it into it, seal it tightly, and stir it with magnetic force to completely dissolve the solid, and there is an anhydrous calcium chloride drying tube on the condensation tube.
[0051] (2) Pipette 2.9 mL of pyridine into the above mixture, stir and place in an ice-water bath.
[0052] (3) Weigh 1.78 g of solid phosgene and dissolve it in 5 mL of chloroform so that the concentration of solid phosgene is 1.2 mol / L, and quickly pour it into the dropping funnel. In an ice-water bath, the solid phosgene solution was slowly added dropwise to the above mixture, stirring rapidly wh...
Embodiment 2
[0057] As described in Example 1, the difference is that polyethylene glycol 4000 is used instead, and 4.00g is dropped into to obtain 3.70g of water-soluble polyethylene glycol (PEG)-based hydroxycamptothecin (HCPT) derivative solid product. It was 60.21%, and the weight average molecular weight was 22500.
Embodiment 3
[0059] As described in Example 1, the difference is that 1.09 g of hydroxycamptothecin (HCPT) is dropped into, and 3.56 g of BTC is dropped into to obtain 6.73 g of water-soluble polyethylene glycol (PEG) hydroxycamptothecin (HCPT) derivative solid product. g, the yield is 63.18%, and the weight average molecular weight is 30800.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com