Mesoporous rare earth phosphate fluophor and preparation method thereof
A rare-earth phosphate and rare-earth nitrate technology, applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, luminescent materials, etc., can solve problems such as poor sample order, difficult synthesis of mesoporous rare earth compounds, and unfavorable entry of active molecules , to achieve the effect of large specific surface area, excellent photoluminescence performance, simple and practical method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
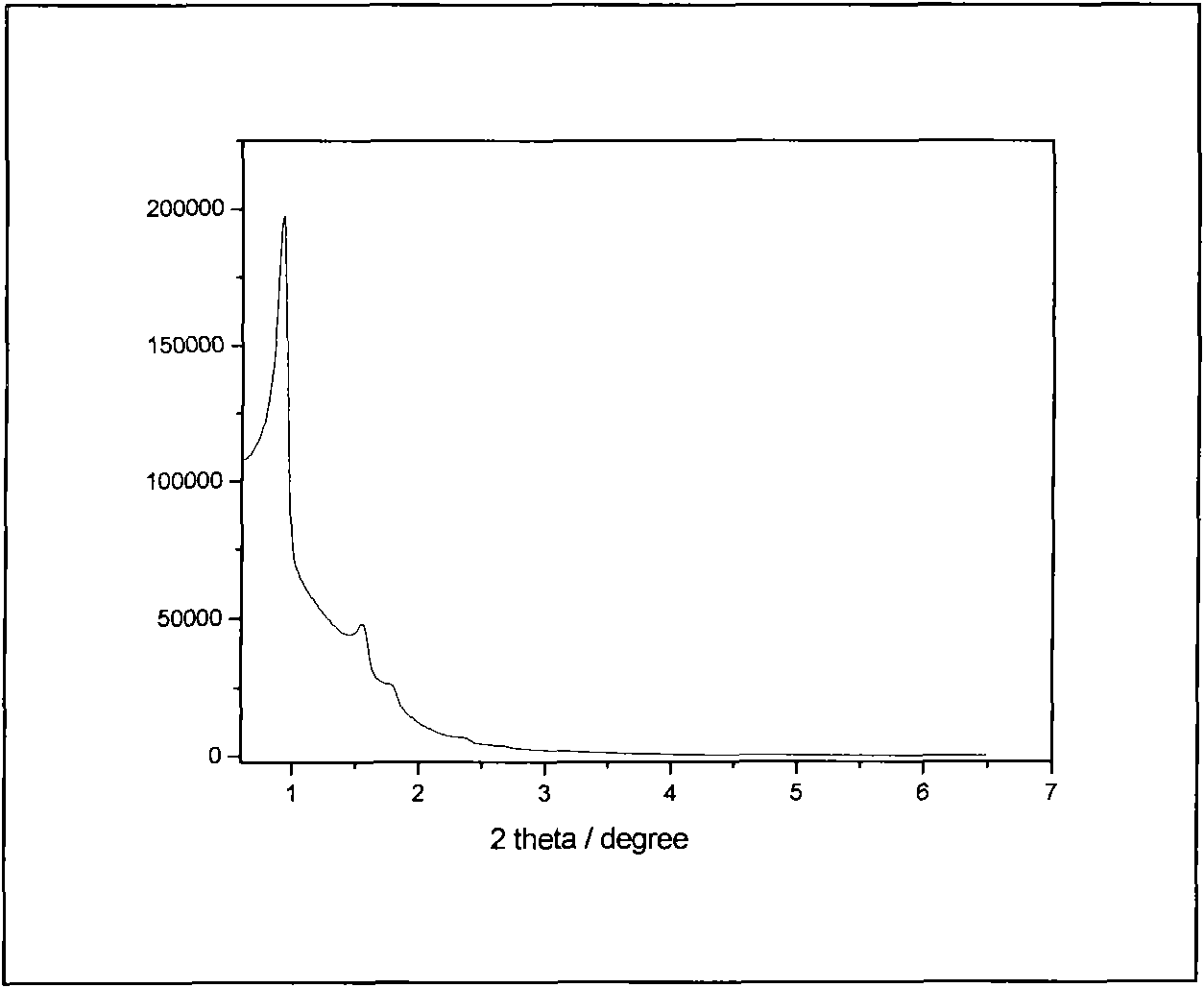
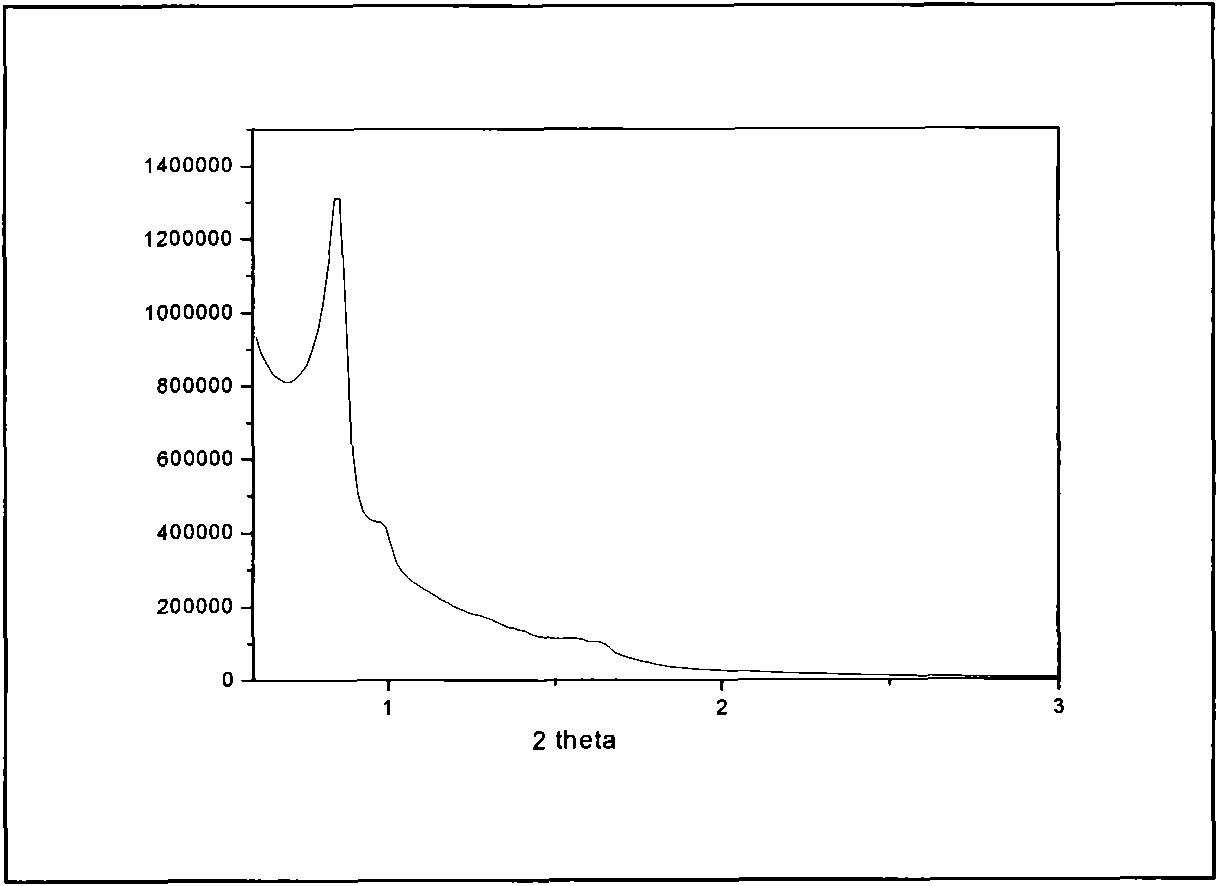
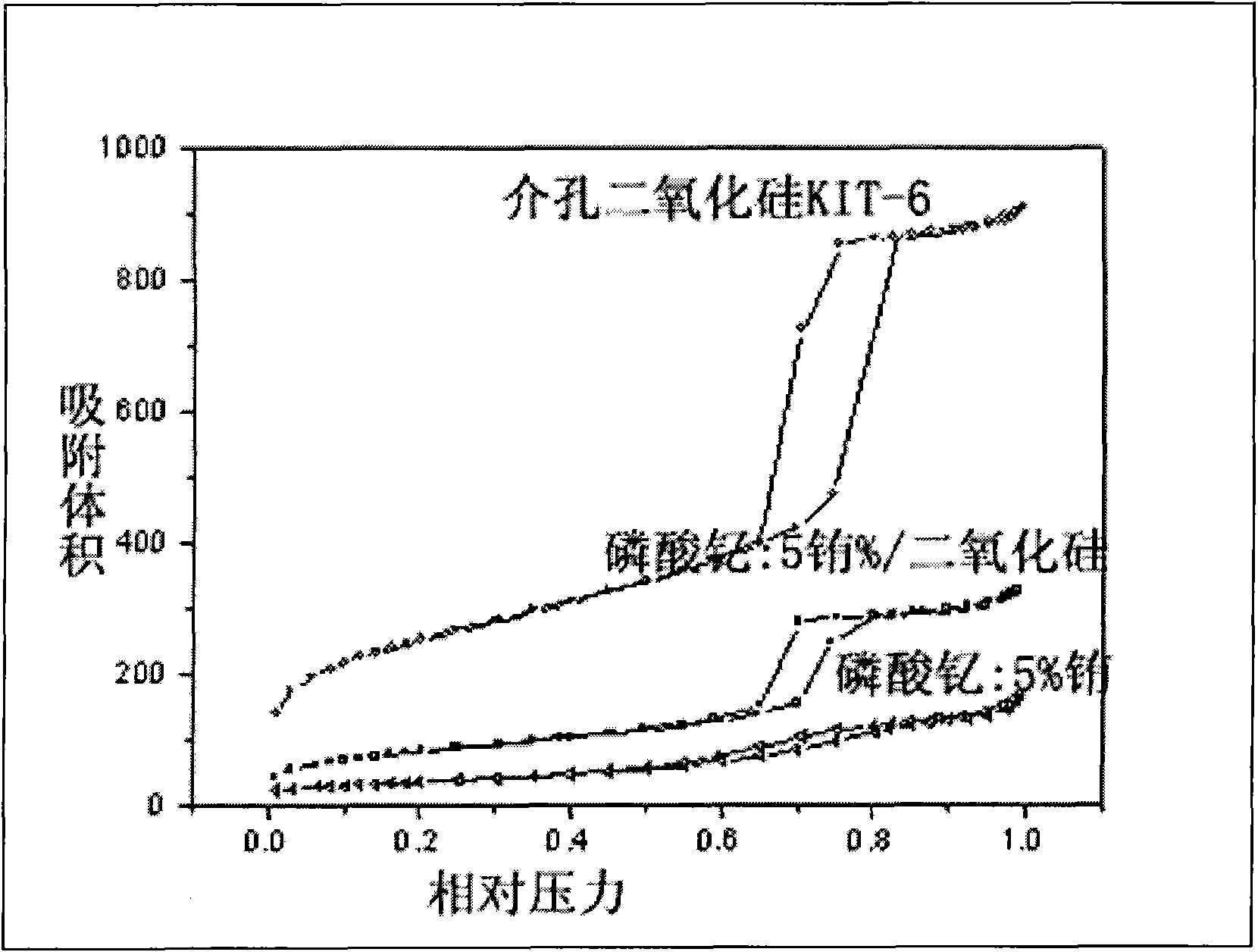
[0052] Disperse 2.0 g of mesoporous silica KIT-6 in 50 ml of n-hexane and fully stir for 30 min, then gradually drop in 2.18 g of Y(NO 3 ) 3 ·6H 2 O, 0.067 g Eu(NO 3 ) 3 ·6H 2 O and 0.6 g concentrated phosphoric acid homogeneous solution. Continue to stir, control the stirring speed to 200-600r / min until the solvent evaporates to dryness, and further place it in an oven at 100°C for 24h. Put this rare earth yttrium phosphate: europium / KIT-6 compound in a muffle furnace, slowly raise the temperature to 650°C, keep it for 5 hours, stop heating, and take it out after natural cooling. Stir with 30ml of 4% hydrofluoric acid for 15 minutes, control the stirring speed to 200-600r / min, further centrifuge, discard the supernatant, repeat 3 times to remove the silica mesoporous hard template, and finally use deionized water Continue washing until the pH of the filtrate is neutral, filter and dry. Thus, mesoporous YPO with three-dimensional cubic Ia3d structure was obtained 4 :Eu...
Embodiment 2
[0054] Disperse 1.0 g of mesoporous silica SBA-15 in 45 ml of n-hexane and stir well for 1 h, then gradually drop in 1.1 g of Y(NO 3 ) 3 ·6H 2 O, 0.035 g Eu(NO 3 ) 3 ·6H 2 O and 0.6 g concentrated phosphoric acid homogeneous solution. Continue to stir, control the stirring speed to 200-600r / min until the solvent evaporates to dryness, and further place it in an oven at 100°C for 24h. Put this rare earth yttrium phosphate: europium / KIT-6 compound in a muffle furnace, slowly raise the temperature to 650°C, keep it for 5 hours, stop heating, and take it out after natural cooling. Use 2mol.L at 40°C -1 NaOH40ml was stirred for 40min at a stirring speed of 200-600r / min, further centrifuged, discarded the supernatant, repeated 4 times to remove the silica mesoporous hard template, and finally continued to wash with deionized water until the pH of the filtrate showed neutral. Filter and dry. Mesoporous YPO with a two-dimensional hexagonal structure P6mm 4 :5%molEu 3+ nanop...
Embodiment 3
[0056] Disperse 1.0 g of mesoporous silica KIT-6 in 45 ml of n-hexane and stir well for 1 h, then gradually drop in 1.1 g of Y(NO 3 ) 3 ·6H 2 O, 0.068 g Tb(NO 3 ) 3 ·6H 2 O and 0.6 g concentrated phosphoric acid homogeneous solution. Continue to stir, control the stirring speed to 200-600r / min until the solvent evaporates to dryness, and further place it in an oven at 100°C for 24h. Put this rare earth yttrium phosphate: terbium / KIT-6 compound in a muffle furnace, slowly raise the temperature to 650°C, keep it for 5 hours, stop heating, and take it out after natural cooling. Stir with 40ml of 3% hydrofluoric acid for 20min, and control the stirring speed to 200-600r / min. Further centrifugation, discarding the supernatant, repeated 3 times to remove the silica mesoporous hard template, and finally continued washing with deionized water until the pH of the filtrate was neutral, filtered, and dried. Thus, mesoporous YPO with three-dimensional cubic Ia3d structure was obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com