3-carbonyl-6-ethoxycarbonyl-thiazole pyrimidine compound and synthesis method and application thereof
An ethoxyformyl and thiazolo technology is applied in the fields of 3-carbonyl-6-ethoxyformyl-thiazolopyrimidine compounds and synthesis, can solve the problems such as few reports on synthesis, and achieves concise preparation method, novel structure, Effects that are easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Synthetic steps and structural confirmation of intermediate (1)
[0039]
[0040] Step 1: Mix 2.88g of ethyl benzoyl acetate, 1.36g of thiourea, 2.04g of p-methoxybenzaldehyde, 675mg of stannous chloride, and 15ml of absolute ethanol, and stir and reflux for 8h. Cool to room temperature, add 200ml of ice water, stir until a white solid precipitates, filter, and recrystallize with absolute ethanol to obtain 4g (yield 72%).
[0041] Step 2: 736mg of the product of step 1, 189mg of chloroacetic acid, 164mg of sodium acetate, 12ml of acetic acid and acetic anhydride (volume ratio 3:1) were stirred and refluxed for 7h. Cool to 25° C., add 200 ml of ice water until a yellow solid precipitates, filter, and recrystallize with methanol to obtain 740 mg (90% yield) of intermediate (1).
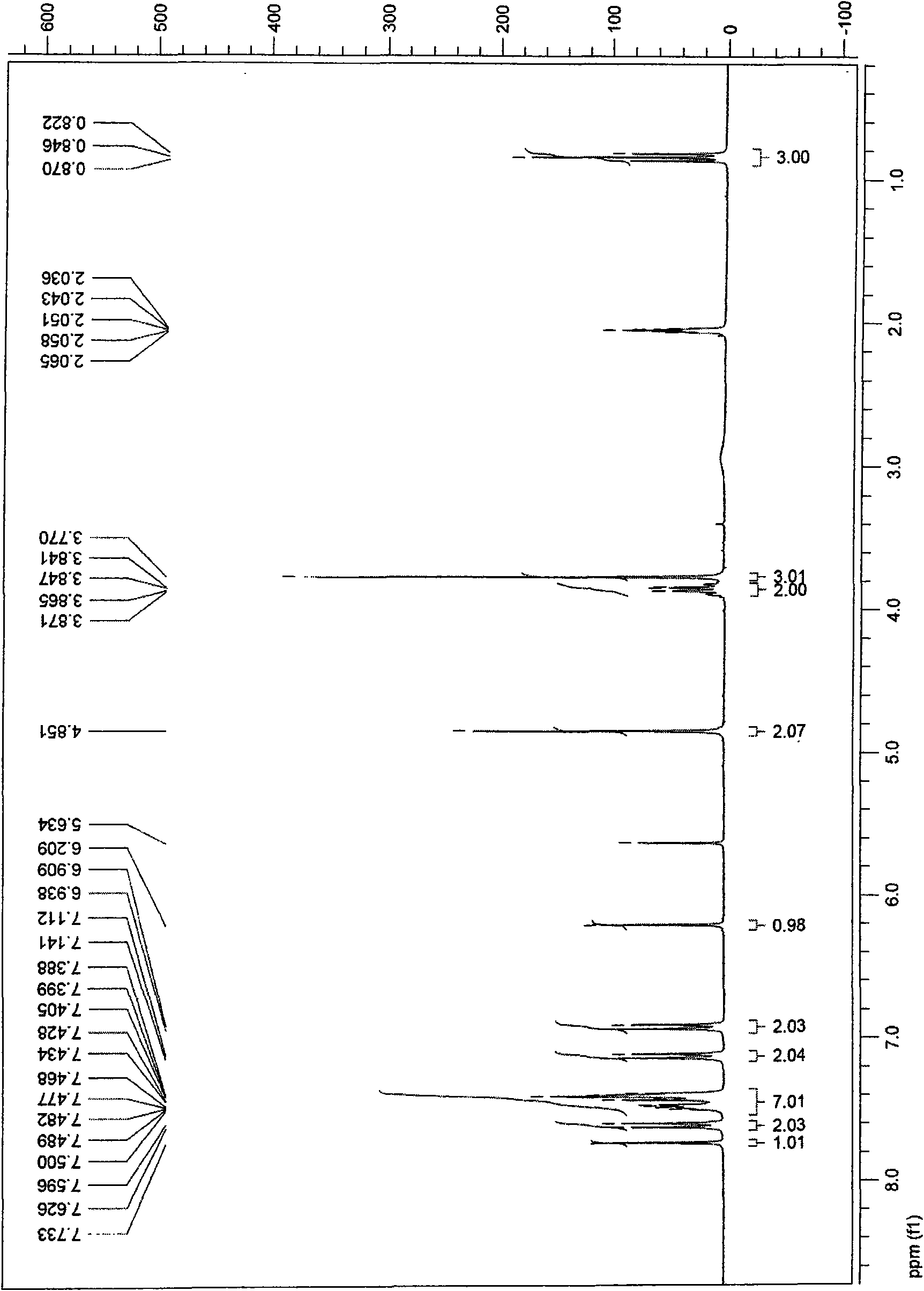
[0042] 1 H-NMR (CDCl 3 )δ6.85~7.43(m, 9H), 3.73~3.92(m, 7H), 0.84(t, 3H).
Embodiment 2
[0043] Embodiment 2: Synthetic steps and structural confirmation of intermediate (2)
[0044]
[0045]Step 1: Mix 2.88g of ethyl benzoyl acetate, 1.36g of thiourea, 2.25g of piperonal, 675mg of stannous chloride, and 15ml of absolute ethanol, and stir to reflux for 6h. Cool to room temperature, add 250ml of ice water, stir until a white solid precipitates, filter, and recrystallize with absolute ethanol to obtain 3.6g (yield: 65%).
[0046] Step 2: 764mg of the product of step 1, 189mg of chloroacetic acid, 164mg of sodium acetate, 12ml of acetic acid and acetic anhydride (volume ratio 1:1) were stirred and refluxed for 9h. After cooling to room temperature, 200 ml of ice water was added to precipitate a yellow solid, which was filtered and recrystallized from methanol to obtain 700 mg (85% yield) of intermediate (2).
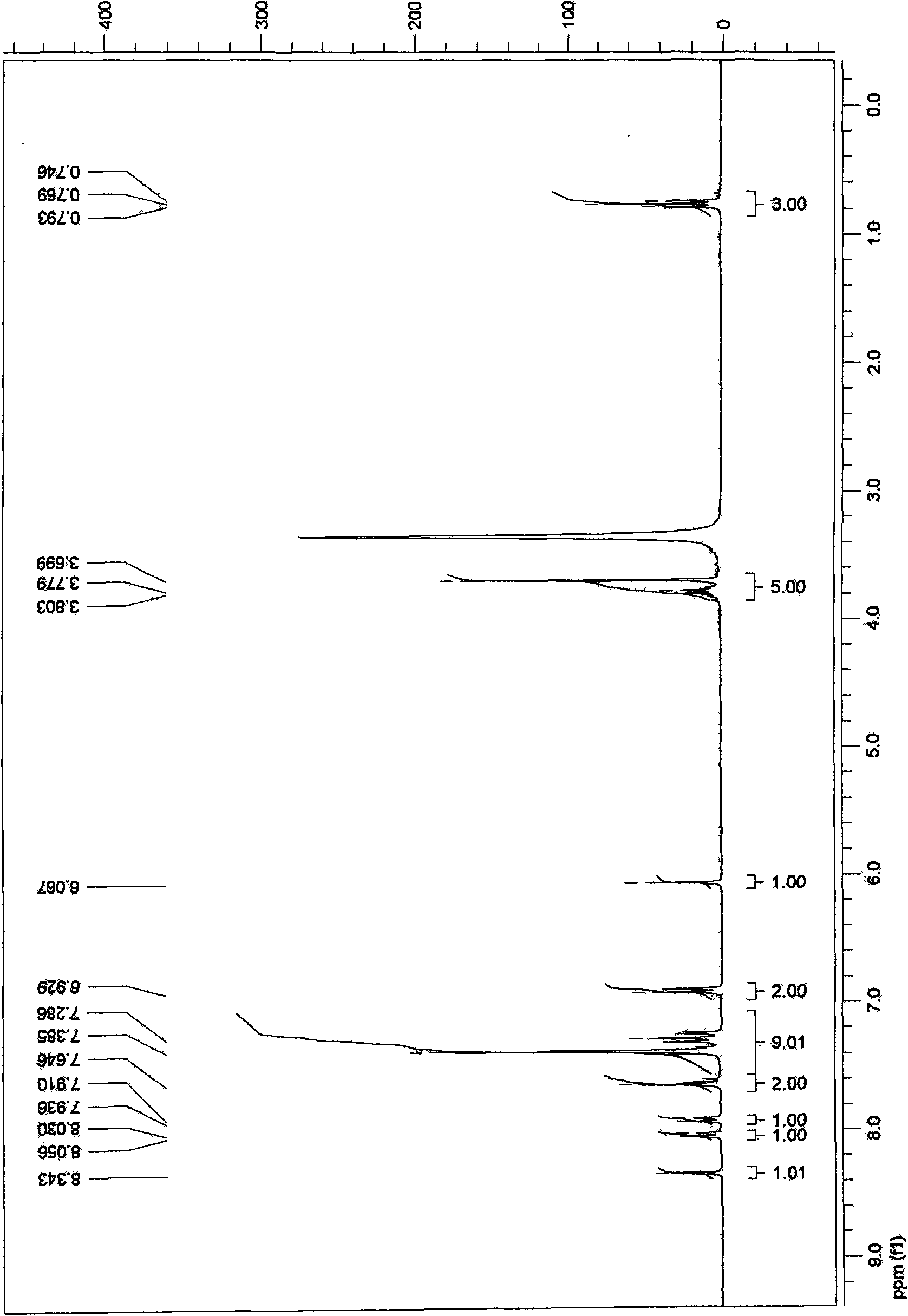
[0047] 1 H-NMR (CDCl 3 )δ6.10~7.44(m, 8H), 5.95(s, 2H), 3.75~3.92(m, 4H), 0.86(t, 3H).
Embodiment 3
[0048] Example 3: Synthesis steps and structural confirmation of 3-(2-(5-methoxyfuryl)) benzoate
[0049] Step 1: Add 2.05g of anthranilic acid to 24ml of water and 8ml of concentrated hydrochloric acid, add 7ml of sodium nitrite aqueous solution (0.18g / ml) to it, and stir at 0°C for 0.5h. Add 10ml of furfural in acetone (0.14g / ml) and 5ml of copper chloride aqueous solution (0.17g / ml), stir at room temperature for 24h, filter with suction, wash with hot water, and dry to obtain 2.4g of yellow solid (yield 73 %).
[0050] Step 2: 1g of the product of step 1, add 10ml of methanol and a catalytic amount of concentrated sulfuric acid, stir and reflux for 4h, stop the reaction, extract with ethyl acetate, flash column chromatography (ethyl acetate:petroleum ether=1:4) to obtain 250mg of viscous Liquid (25% yield).
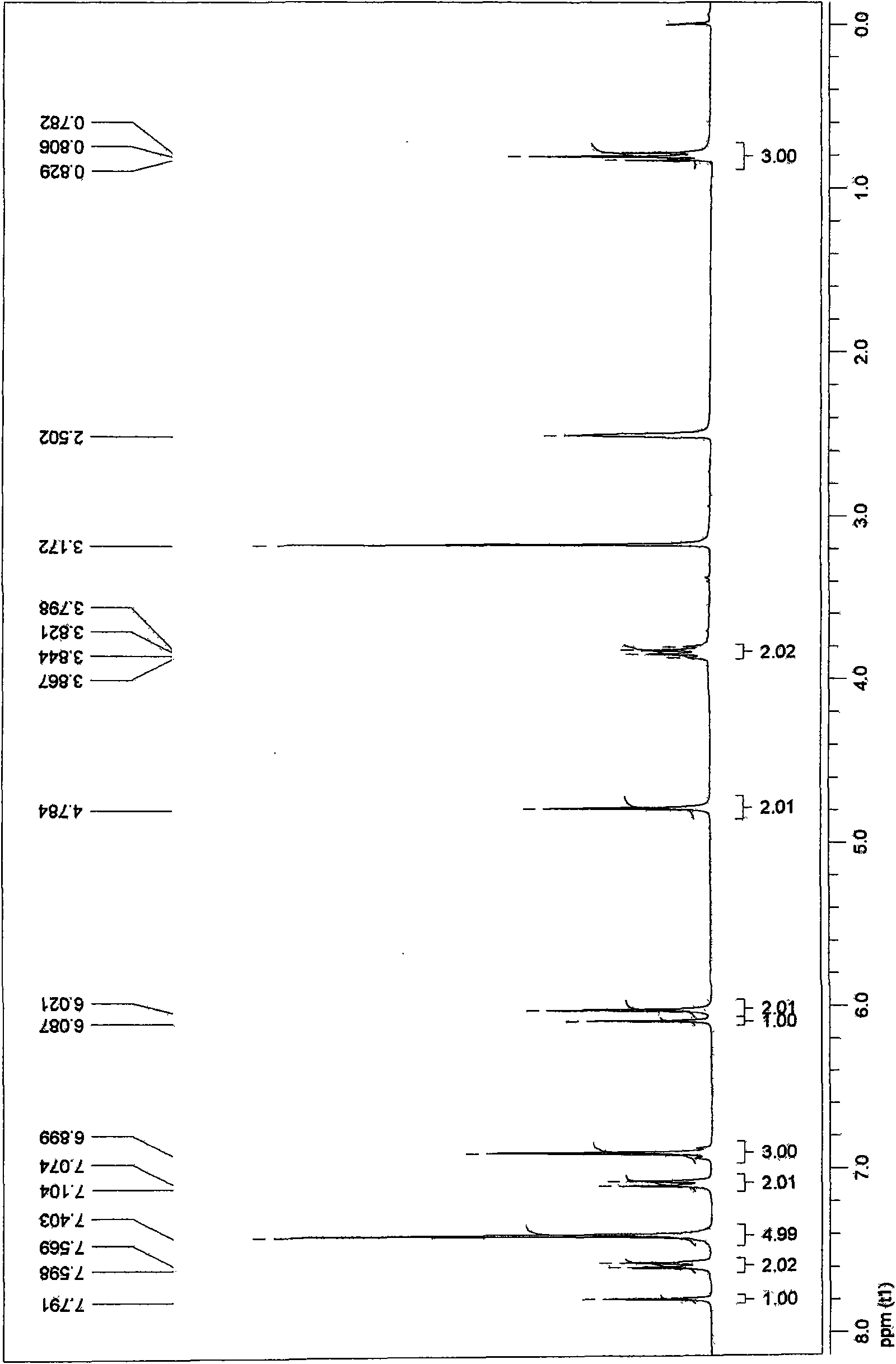
[0051] 1 H-NMR (CDCl 3 )δ9.55(s, H), 6.80~8.30(m, 6H), 3.84(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com