Anti-tumor predrug by taking novel amphipathic dendritic macromolecules as carriers based on glycerol as branching unit and preparation method thereof
A branching unit and dendritic technology, applied in the field of anti-tumor prodrugs and preparation, to achieve the effect of improving hydrophilicity, improving degradability, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
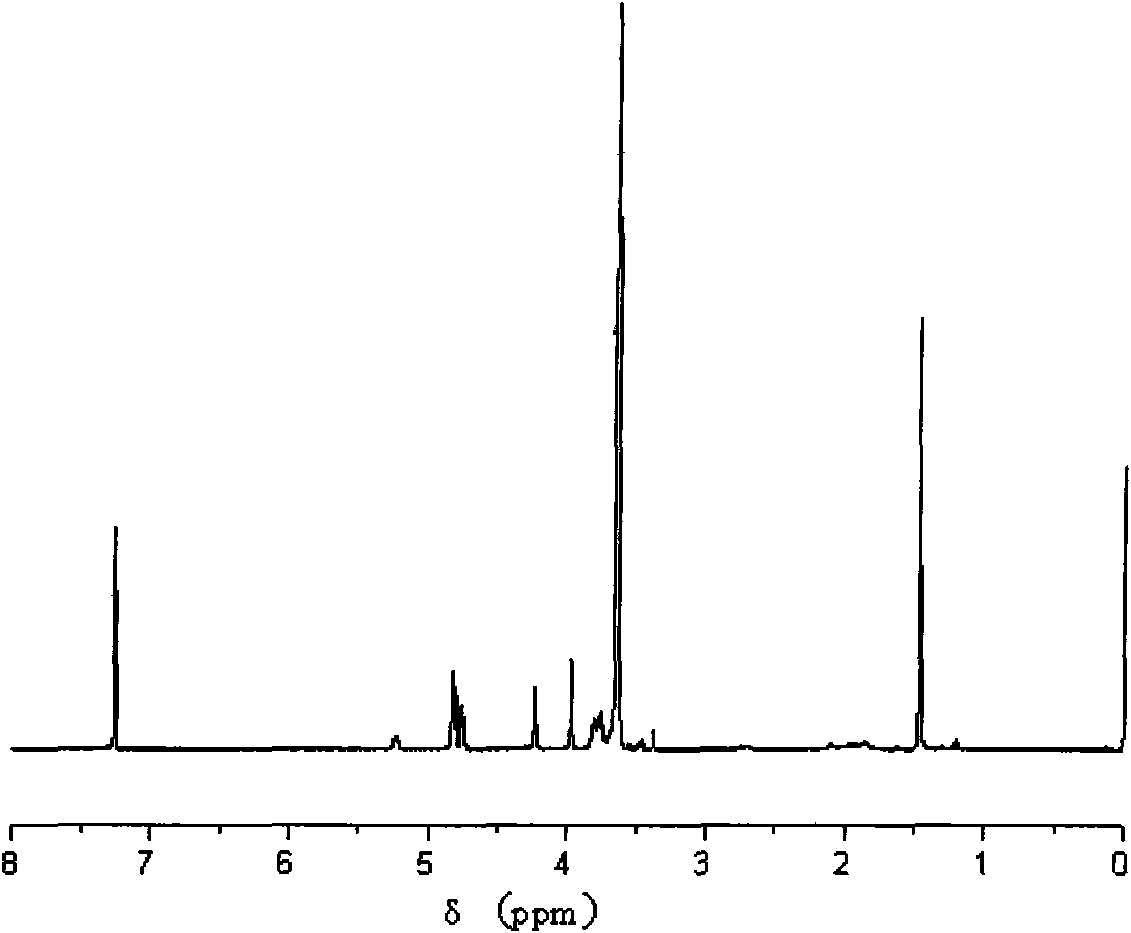
[0031] Example 1: Preparation of an amphiphilic dendrimer paclitaxel prodrug containing a glycolic acid oligomer.
[0032] a) Add 9g of glycolic acid and 350mL of anhydrous ether into a 500ml single-necked round bottom flask with magnetic stirring. After the glycolic acid is completely dissolved, add 5.69mL of benzyl bromide and 12g of catalyst, and react for 24 hours. After the reaction was completed, it was filtered, and the filtrate was concentrated with a rotary evaporator. After extraction, 8.5 g of the product was obtained, with a yield of 80.7%.
[0033] b) Add 136g of benzaldehyde, 102g of glycerol, 20ml of cyclohexane and a catalyst into a 500ml single-necked flask with magnetic stirring and water separator, react at 100°C for 4.5h, pour off the upper layer of cyclohexane, and add 150ml of dihydrogen to the system. Chloromethane was extracted three times with alkaline aqueous solution, saturated NaCl solution and water respectively. Dry over anhydrous magnesium sulfa...
Embodiment 2
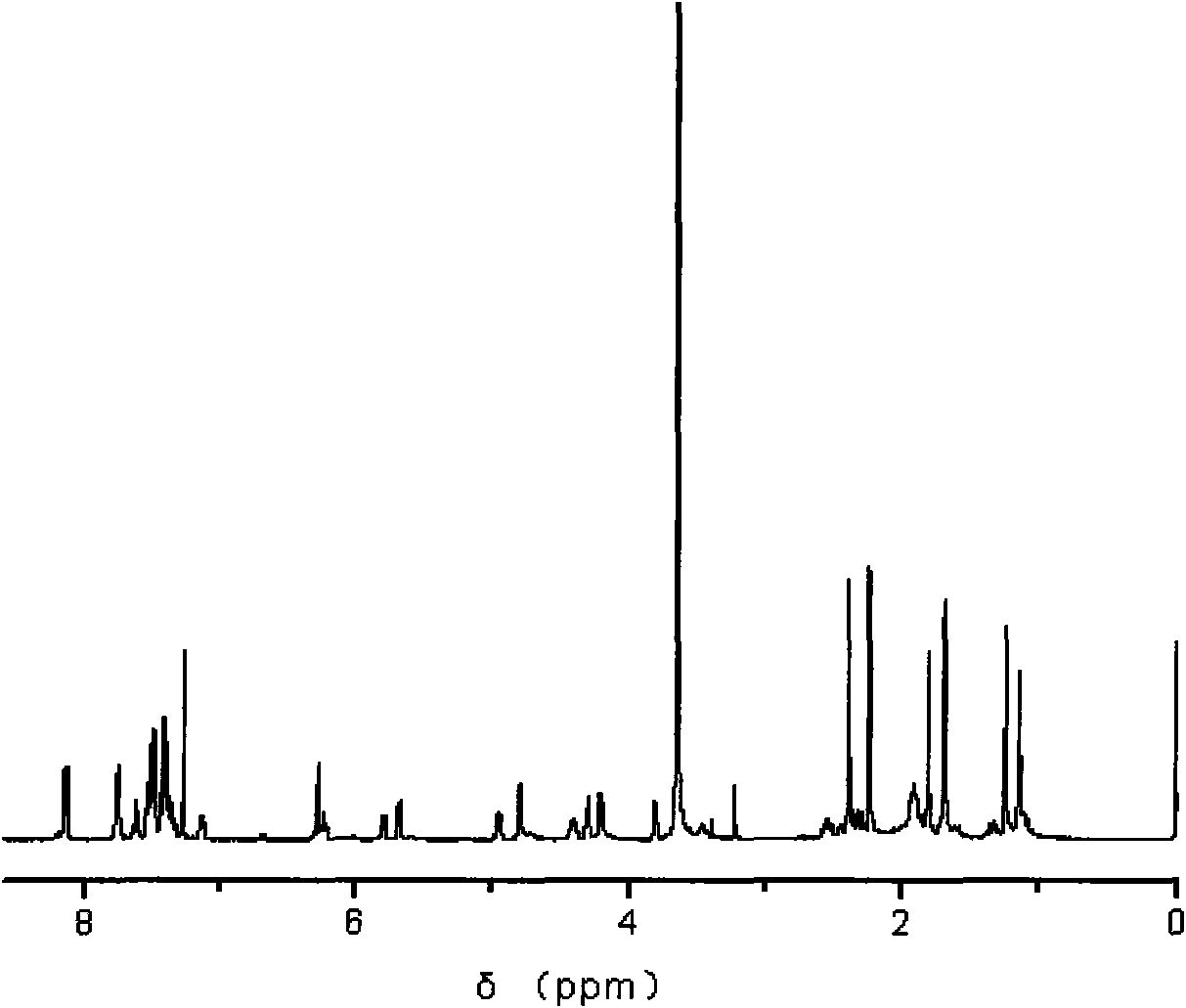
[0040] Example 2: Preparation of an amphiphilic dendrimer paclitaxel prodrug containing alternating oligomers of glycolic acid and lactic acid.
[0041] a) 2g 2-benzyloxypropanediol, 6g tert-butyl bromopropionate, 50ml dichloromethane, and 1.6g base were added in a 250ml single-mouthed round-bottomed flask with a magnetic stirring device, and after 24 hours of reaction, the precipitate was filtered off, Concentrate the solvent by rotary evaporation, use petroleum ether: ethyl acetate as the developing solvent, and separate through the column. A monomer in which the benzyl group protects the hydroxyl group and the tert-butyl group protects the carboxyl group is obtained.
[0042] b) Add 5g of lactic acid and 3.8g of alkali into a 250ml single-necked flask with magnetic stirring and condensing reflux device. After 4 hours of salt formation reaction, add 5.6g of tert-butyl bromoacetate. After 24 hours of reflux reaction, filter the precipitate generated by the reaction . Concen...
Embodiment 3
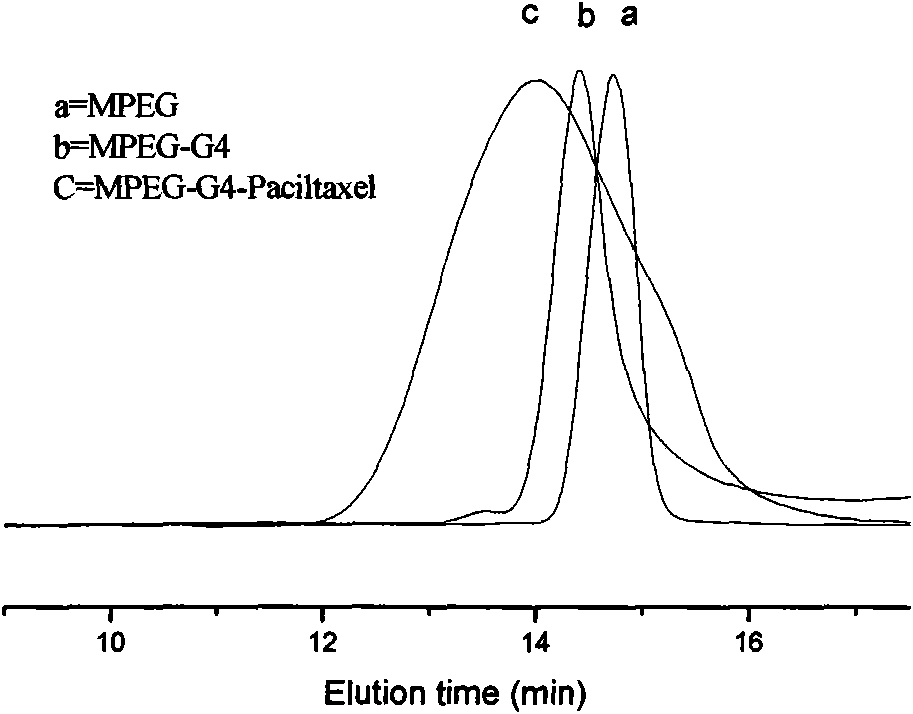
[0045] Example 3: Preparation of an amphiphilic dendrimer paclitaxel prodrug containing alternating oligomers of glycolic acid and glycine.
[0046] a) Preparation of glycolic acid glycine oligomer (taking tetramerization as an example): add an equivalent amount of fluorenylmethoxycarbonyl (Fmoc)-protected glycine and tert-butyl bromoacetate, 100ml anhydrous tetrahydrofuran in a 250ml single-necked flask, After completely dissolving, add an equimolar amount of alkali to form a salt, and a catalyst, and reflux the reaction. After the reaction was completed, it was separated by column chromatography to obtain a white solid (glycolic acid glycine dimer with tert-butyl-protected carboxyl group, Fmoc-protected amino group). Take 5g of each of these dimers, and use 100m piperidine / DMF solution to remove the Fmoc group and glycolic acid glycine dimer respectively.
[0047] b) The same amount of glycolic acid glycolic acid glycine dimer and bromoacetic acid described in a are dissolv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com