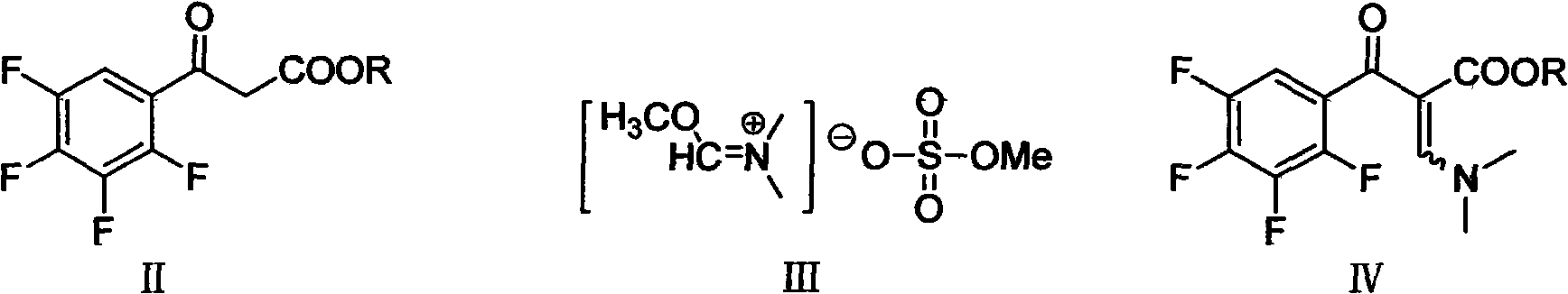Novel method for preparing marbofloxacin
A technology of marbofloxacin and enamine, which is applied in the chemical field, can solve the problems of high price, no industrial value, and harsh reaction conditions of tetrabutyl fluoride ammonium, which is conducive to large-scale preparation and increases the electron cloud density , less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The synthesis of embodiment 1,3-(dimethylamino)-2-(2,3,4,5-tetrafluorobenzoyl) ethyl acetate
[0054] Drop into 4g sodium hydride (60%, 0.10mol) and 200ml toluene in the 500ml four-necked flask that is full of nitrogen, drop the toluene solution of 2,3,4,5-tetrafluorobenzoyl ethyl acetate ( 26.42g dissolved in 60ml toluene). After about 2-3 hours, the dripping is completed, and it is kept at 0° C. for 8 hours. Another 39.85 g of 1,1-dimethyl-2-methoxyimine methanesulfonate (0.20 mol) was added dropwise to the above solution at 0°C, and the reaction was incubated at 0-5°C for 15 hours. After the reaction is complete, filter under reduced pressure and bleach the filter cake with an appropriate amount of toluene. Combine the filtrates, wash twice with deionized water, each time with 40ml of deionized water, then dry with anhydrous sodium sulfate, and filter off the desiccant to obtain 3-(dimethylamino)-2-(2,3,4 , 5-tetrafluorobenzoyl) ethyl acetate toluene feed solution...
Embodiment 2
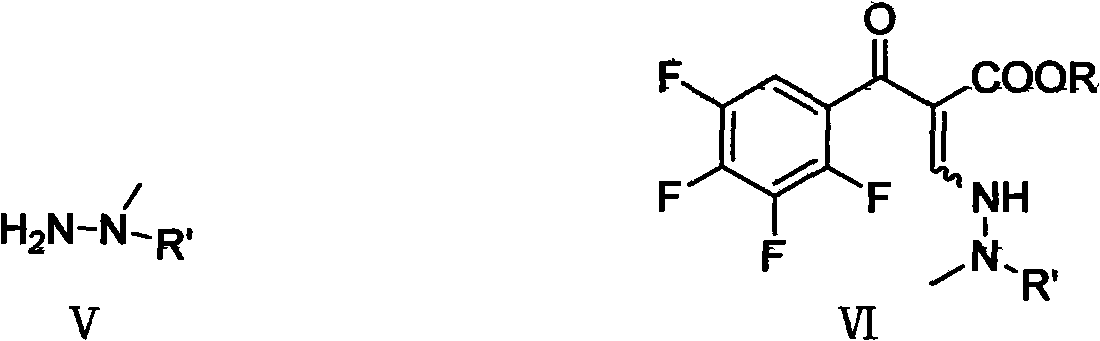
[0055] Embodiment 2, the synthesis of 3-(2-formyl-2-methylhydrazino)-2-(2,3,4,5-tetrafluorobenzoyl ethyl acrylate
[0056] 8.6 ml of glacial acetic acid (0.15 mol) was added to the feed solution obtained in the upward step, stirred for 10 minutes, and 8.81 g of N-amino-N-methylformamide (0.10 mol) was added dropwise at 5°C. After dropping, react at 5-10°C for 6 hours. After the reaction, the pH value was adjusted to 6 with an appropriate amount of sodium hydroxide solution. Then wash with ice water three times, 40ml of ice water each time. The organic layer was dried over anhydrous sodium sulfate, and the desiccant was filtered off to obtain ethyl 3-(2-formyl-2-methylhydrazino)-2-(2,3,4,5-tetrafluorobenzoylacrylate The toluene feed solution was directly used in the next step reaction without purification.
Embodiment 3、6
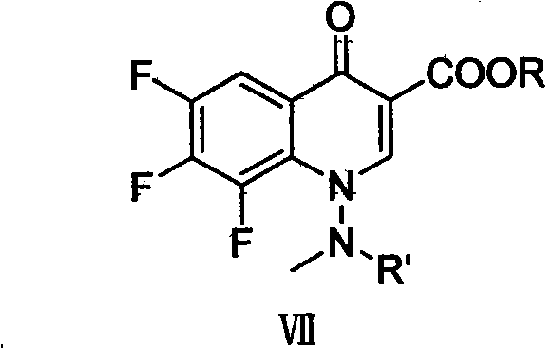
[0057] Embodiment 3,6,7, the synthesis of 8-trifluoro-1-(N-methylformylamino)-4-oxo-1,4-dihydroquinoline-3 carboxylic acid ethyl ester
[0058] Add 6.36g of sodium carbonate (0.06mol) to the feed liquid obtained in the upward step, heat up to reflux, and react under reflux for 4 hours. During the reaction, the product gradually precipitated out. After the reaction, filter with suction. The filter cake was rinsed with deionized water until it became neutral, and then dried under vacuum at 60° C. to obtain 29.54 g (yield 90.0%) of light yellow to yellow powder with a melting point of 188-191° C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com