Method for producing sodium stannate
A production method and technology of sodium stannate, applied in chemical instruments and methods, tin compounds, inorganic chemistry and other directions, can solve the problems of reducing the direct recovery rate of tin, low direct recovery rate of tin, increasing consumption per ton of product, etc., and achieving less impurities , The effect of complete response and fast response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
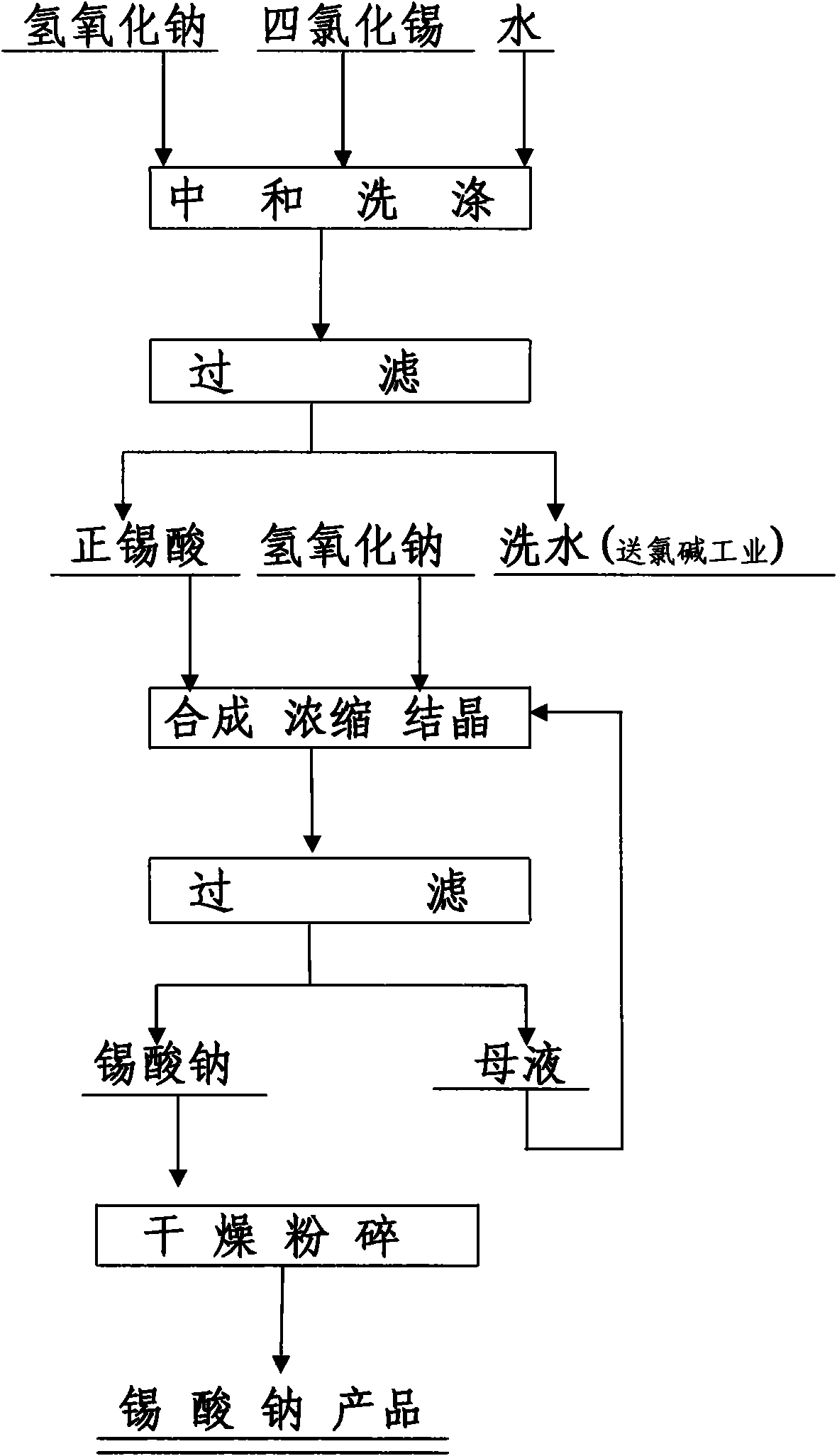
[0024] Under airtight conditions, chlorine gas is added to metal tin, and the chlorination reaction generates tin tetrachloride. Slowly add 600kg of tin tetrachloride to 600kg of water, cool to room temperature, stir, add 800kg of 45% sodium hydroxide aqueous solution to it, and keep stirring and cooling during the neutralization process, keep the reaction temperature at 60℃~80℃, adjust the hydroxide The amount of sodium added to control the neutralization end point pH value of 6-7. After the neutralization operation is completed, 6000kg of water is added each time, and the precipitate is washed 3 times. The washing water produced in the third washing of this tank is used as the second washing water for the precipitation of the next tank, and the washing water generated from the second washing of this tank is used as the first washing water for the precipitation of the next tank. The washing water produced in the first washing is sent to the chlor-alkali industrial electrolysi...
example 2
[0026] Under airtight conditions, chlorine gas is added to metal tin, and chlorination reaction produces tin tetrachloride. Slowly add 600kg tin tetrachloride to 600kg water, cool to room temperature, add it to 800kg weight of 45% sodium hydroxide aqueous solution, and keep stirring and cooling during the neutralization process, and keep the reaction temperature at 60℃~80℃ , Adjust the amount of tin tetrachloride added, and control the neutralization end point to pH 7-8. After the neutralization operation is completed, 6000kg of water is added each time, and the precipitate is washed 3 times. The washing water produced in the third washing of this tank is used as the second washing water for the precipitation of the next tank, and the washing water generated from the second washing of this tank is used as the first washing water for the precipitation of the next tank. The washing water produced in the first washing is sent to the chlor-alkali industrial electrolysis to produce...
example 3
[0028] Take 120kg of ammonium bicarbonate, 240kg of concentrated ammonia water, and 600kg of water, mix, stir, slowly add 300kg of tin tetrachloride, adjust the amount of tin tetrachloride, control the neutralization end point pH value of 6-7, temperature 40℃-60℃. After the neutralization operation is completed, add water to wash the sediment 3 times, add 3000 kg of water each time, stir for 10 minutes, clarify for 4 hours, and discharge the supernatant by siphoning. After the washed precipitate is filtered with a plate and frame filter, orthostannic acid is obtained. Weigh, sample and test the tin content, calculate the weight of tin contained in stannic acid, weigh sodium hydroxide according to the molar ratio of sodium hydroxide to tin 2:1, add to stannic acid, stir and mix, and heat to 85°C , Concentrated crystallization, centrifugal filtration, recycling of crystallization mother liquor, the filter cake is dried and crushed to obtain 330kg of sodium stannate product, conta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com