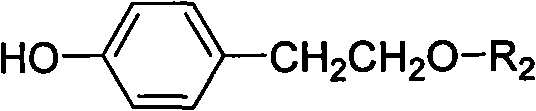
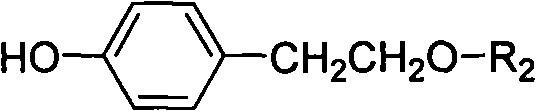
Method for preparing tyrosol glucoside in quantities
A tyrosol glycoside and a large number of technologies are applied in the field of mass preparation of tyrosol glycoside, can solve the problems of increased synthesis cost, complicated synthesis operation, potential safety hazards and the like, and achieve the effects of simple operation, stable process parameters and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Mass preparation of tyrosol-β-D-galactoside
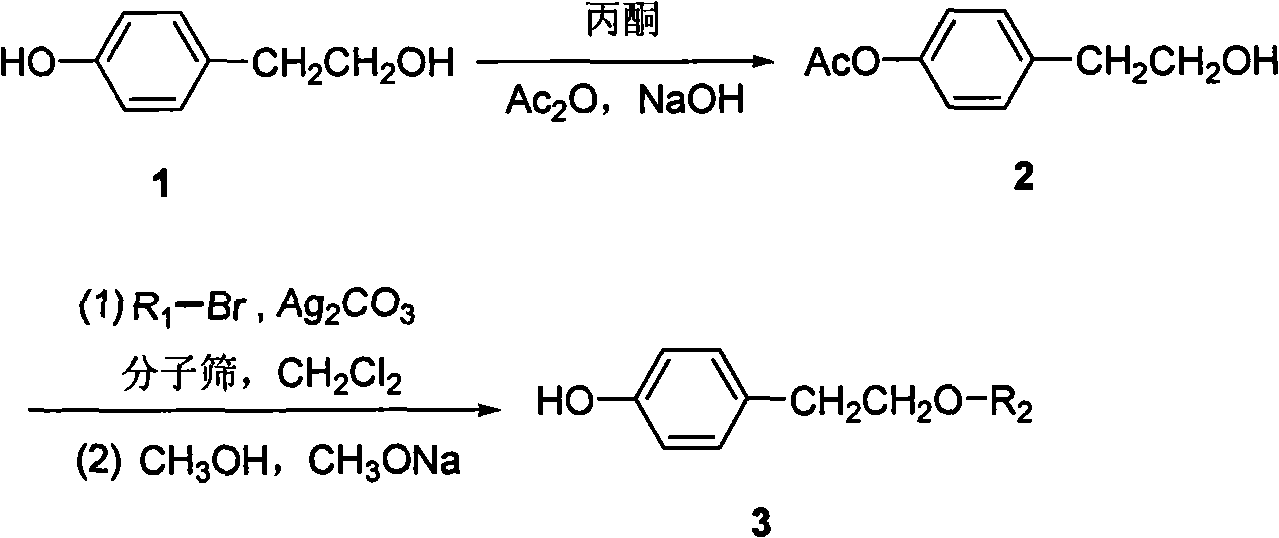
[0020] (1) Synthesis of p-Acetoxyphenethyl alcohol
[0021] Dissolve 1.38 kg (10 mol) of tyrosol in 2 L of acetone, add 0.5 L of saturated aqueous sodium hydroxide solution, slowly add 2 L of acetic anhydride, and stir at room temperature for 4 h. The solvent was removed under reduced pressure, the insoluble matter was dissolved with ethyl acetate, washed with distilled water and saturated brine successively until neutral, and concentrated to obtain a colorless oil. Placed in the freezer overnight, white crystals were formed, filtered and washed to obtain 1.53 kg of product, yield 85%, m.p.39-40°C.
[0022] (2) Synthesis of β-D-1-bromo-tetraacetylgalactose
[0023] 20 mL of HClO 4 Slowly added dropwise to 3L of acetic anhydride at 0°C, raised to room temperature, then added 1.08kg (6mol) D-galactose in batches, and reacted at 20°C for 5h. At this temperature, slowly add 0.31kg (10mol) red phosphorus and 580mL ...
Embodiment 2
[0026] Example 2: Mass preparation of tyrosol-β-D-xyloside
[0027] (1) Synthesis of β-D-1-bromo-triacetylxylose
[0028] 20 mL of HClO 4 Slowly added dropwise to 3L of acetic anhydride at 0°C, raised to room temperature, then added 0.675kg (4.5mol) of D-xylose in batches, and reacted at 30°C for 5h. At this temperature, slowly add 0.23kg (7.5mol) red phosphorus and 435mL Br 2 , After reacting for 2h, add 270mL of H 2 O, continue to stir at room temperature for 2h. Add 1.5L CH 2 Cl 2 , filtered to remove insoluble matter, and the filtrate was washed twice with 1.5L ice water, then added to 2.25L cold saturated NaHCO 3 solution, stirred for 20 min, separated, the organic layer was dried with anhydrous magnesium sulfate, filtered, and the solvent was removed to obtain 1.17 kg of light yellow oil with a yield of 68%. The purity can be directly used in the next reaction.
[0029] (2) Synthesis of tyrosol-β-D-xyloside
[0030] The synthetic method of p-acetoxyphenethyl alco...
Embodiment 3
[0032] Embodiment 3: the synthesis of p-Acetoxyphenethyl alcohol (comparative example)
[0033] Dissolve 1.38kg (10mol) of tyrosol in 2L of isopropanol, add 0.5L of saturated aqueous sodium hydroxide solution, and slowly add 2L of acetic anhydride, the reaction system gradually becomes a viscous paste, and the temperature of the reaction system rises significantly, continue to stir 6-8h. The solvent was removed under reduced pressure, the insoluble matter was dissolved with ethyl acetate, washed with distilled water and saturated brine successively until neutral, and concentrated to obtain a colorless oil. 0.58 kg of the product was isolated by column chromatography with a yield of 32%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com