Human interferon alpha derivatives and preparation and use of pegylated products thereof
A technology of pegylation and interferon α, applied in the field of biomedicine, can solve the problems of rapid plasma clearance, unsatisfactory clinical treatment effect, and large side effects, and achieve the effect of treating or preventing viral infection or tumor disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
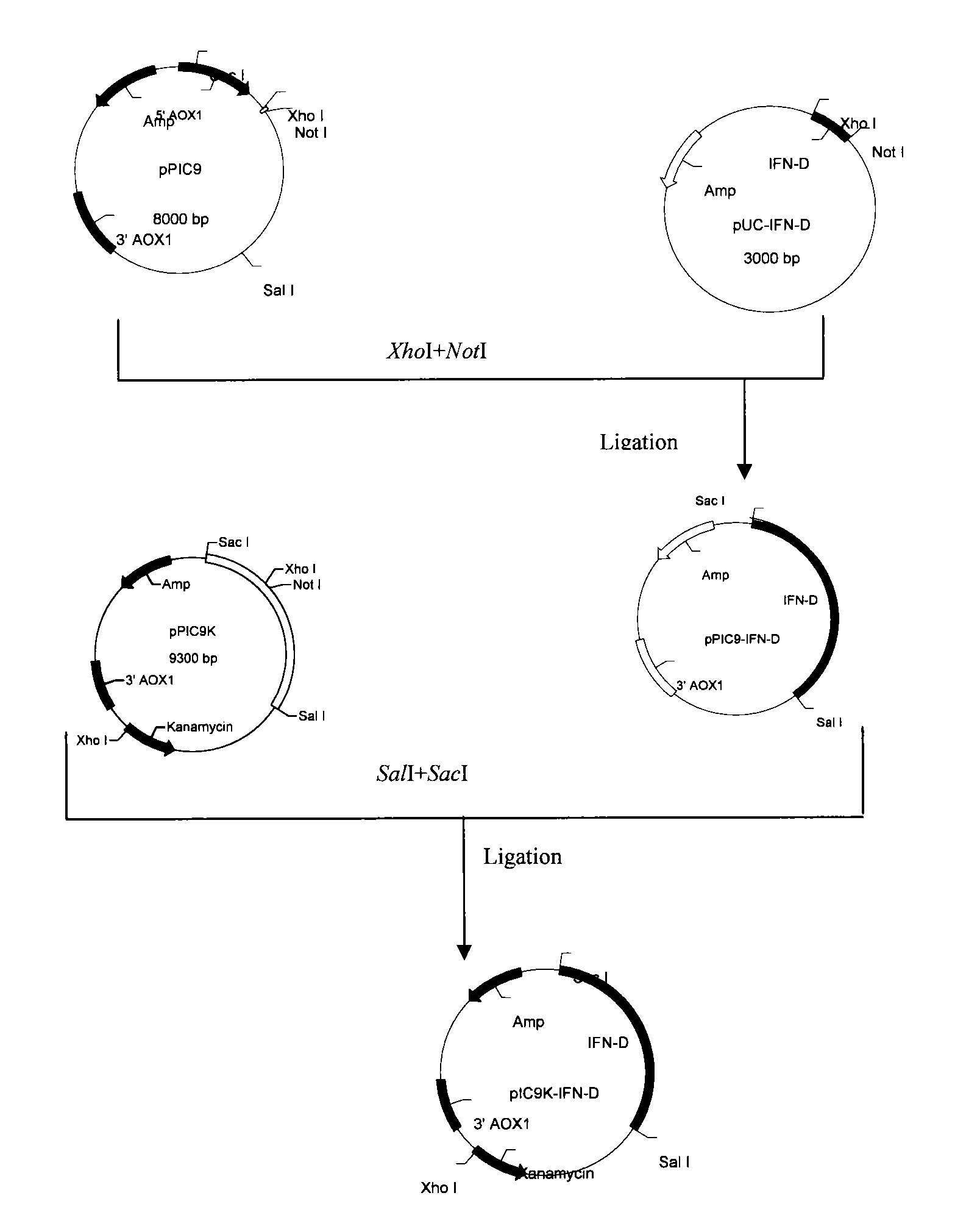
[0035] Example 1 Secretion and expression of Glu-Phe-Met-IFNα-2a in methanolic yeast
[0036] 1. Acquisition and design of target genes
[0037] The amino acid sequence of Glu-Phe-Met-IFNα-2a (hereinafter referred to as IFN-D) is shown in SEQ ID NO: 1, and the sequence length is 168 amino acids.
[0038] After obtaining the cDNA of IFNα-2a through Genebank, the corresponding codons were changed to yeast preference. And the corresponding nucleotide sequence of Glu-Phe-Met was added at the N-terminus. The cDNA sequence was used to construct the PIC9K expression plasmid of Pichia pastoris. The constructed expression plasmid was transformed into GS115 host bacteria to achieve secretory expression. So adding KEX to the design 2 The enzyme recognition site CTC GAG AAA AGA, wherein CTC GAG is the XhoI restriction site. At the same time, a double stop codon TGA TAA and a Not I restriction restriction sequence GCG GCCGC were introduced into the 3' end. SEQ ID NO: 2 is the cDNA se...
Embodiment 2I
[0047] The purification of embodiment 2IFN-D
[0048] 1. Cationic gel column (such as CM Sepharose F.F. gel) chromatography:
[0049] The pH 3.8-4.6 acetate buffer was used for column loading and elution, and electrophoresis monitoring was used to collect the target substance. Then, the target substance was dialyzed with a pH 7.5-8.5 Tris-HCl buffer solution.
[0050] 2. Anion gel column (such as DEAE Sepharose F.F. gel) chromatography:
[0051] The pH7.5-8.5 Tris-HCl buffer solution is used for column loading and elution, and the target substance is collected. Then dialyze the target substance with pH 7.5-8.5 phosphate buffer solution.
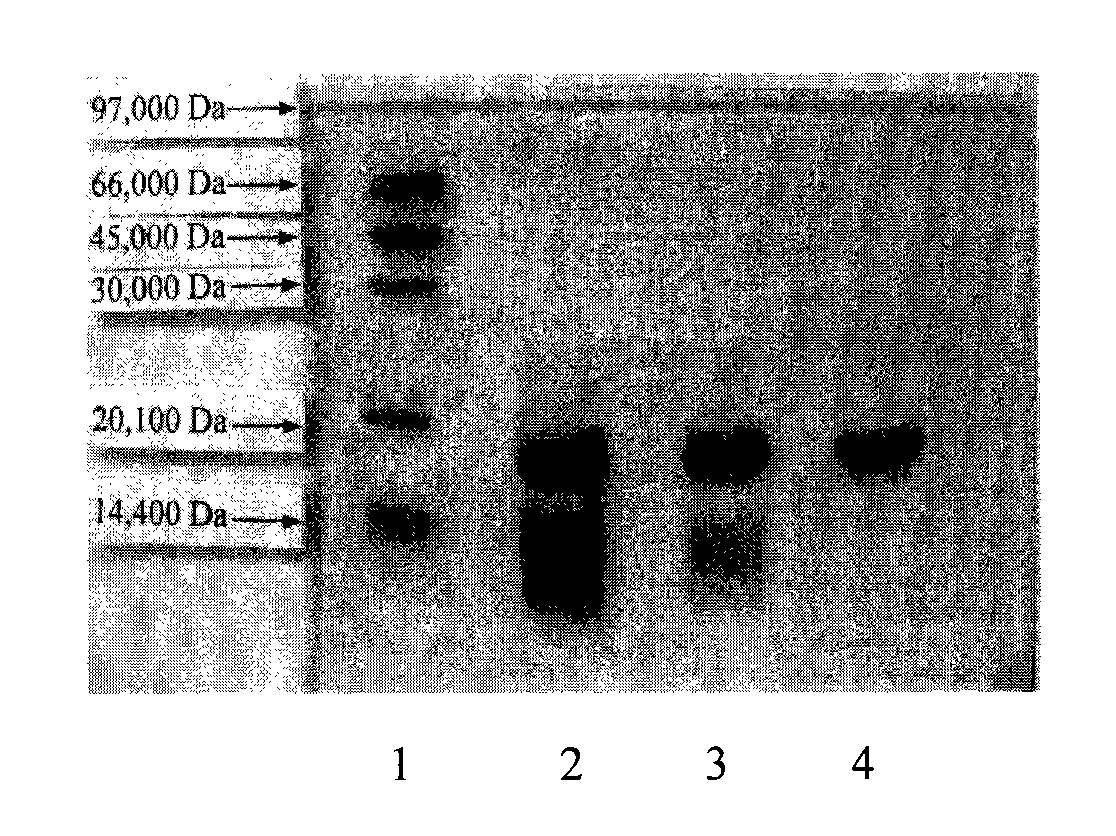
[0052] 3. SDS-PAGE detection: The pass-through solution and the target peak of the above CM column and DEAE column were taken for detection. The test results showed that the fermentation broth was purified by the above column chromatography, and IFN-D with a purity of more than 95% was obtained. see attached results figure 2 shown.
Embodiment 3
[0053] Example 3 Preparation and purification process of PEG coupling modification sample:
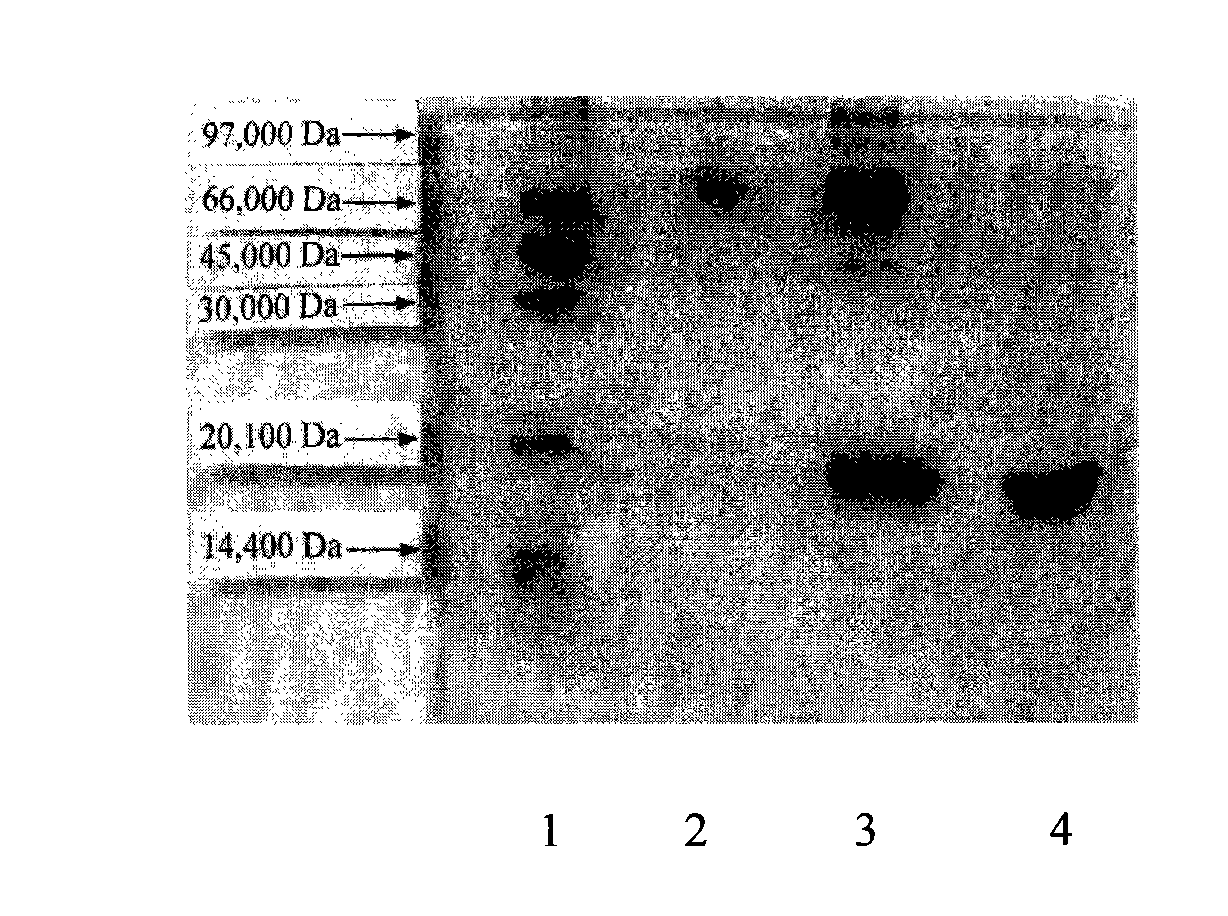
[0054] 1. Dialyze the IFN-D sample with phosphate buffer, then add equimolar ALD-PEG 20KD for modification at 2-15°C, and the reaction time is 24-36 hours. The obtained modified sample was tested by SDS-PAGE. The test results showed that after PEG coupling modification, the molecular weight increased from the original 19,000 Daltons to nearly 40,000 Daltons, and the modification rate reached more than 40%, and the target compound was obtained mPEG(20KD)-IFN-D. The results are attached image 3 shown.
[0055] 2. Purification of mPEG(20KD)-IFN-D cationic gel column (such as SP Sepharose F.F. gel):
[0056] Adjust the conductivity of the modified sample to 4.0-5.0 to terminate the reaction, and put it on the SP column for purification. Use acetate buffer for elution to collect target compounds. Dialyze the target substance against a phosphate buffered saline solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com