Conjugated polymer based on condensed ring thiophene and diazosulfide as well as preparation method and application thereof
A technology of benzothiadiazole and conjugated polymers, which is applied in the field of conjugated polymers based on fused ring thiophene and benzothiadiazoles and their preparation and application, and can solve the problems of large gaps, no absorption, and hole migration Low efficiency and other problems, to achieve the effect of good processability and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
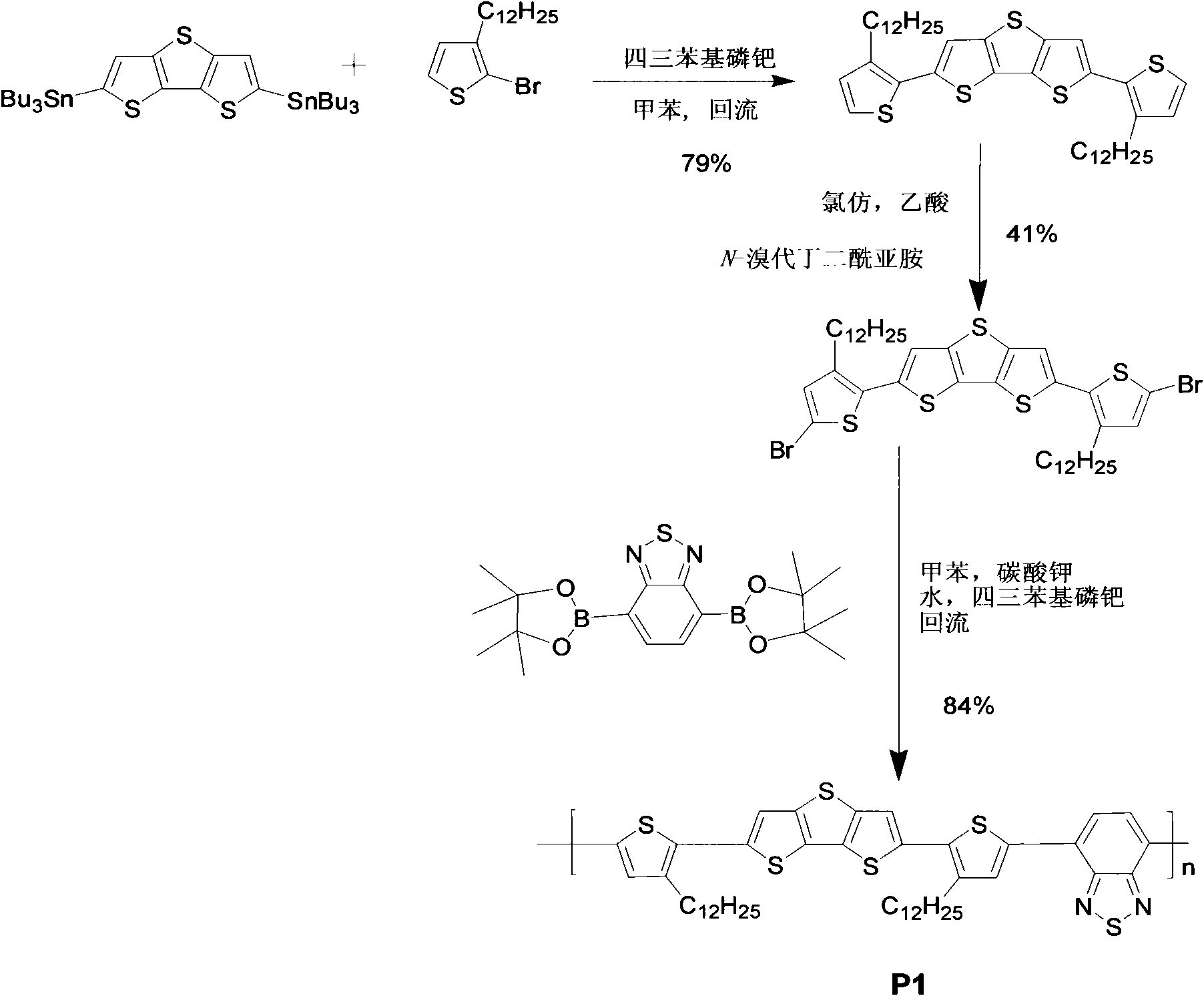
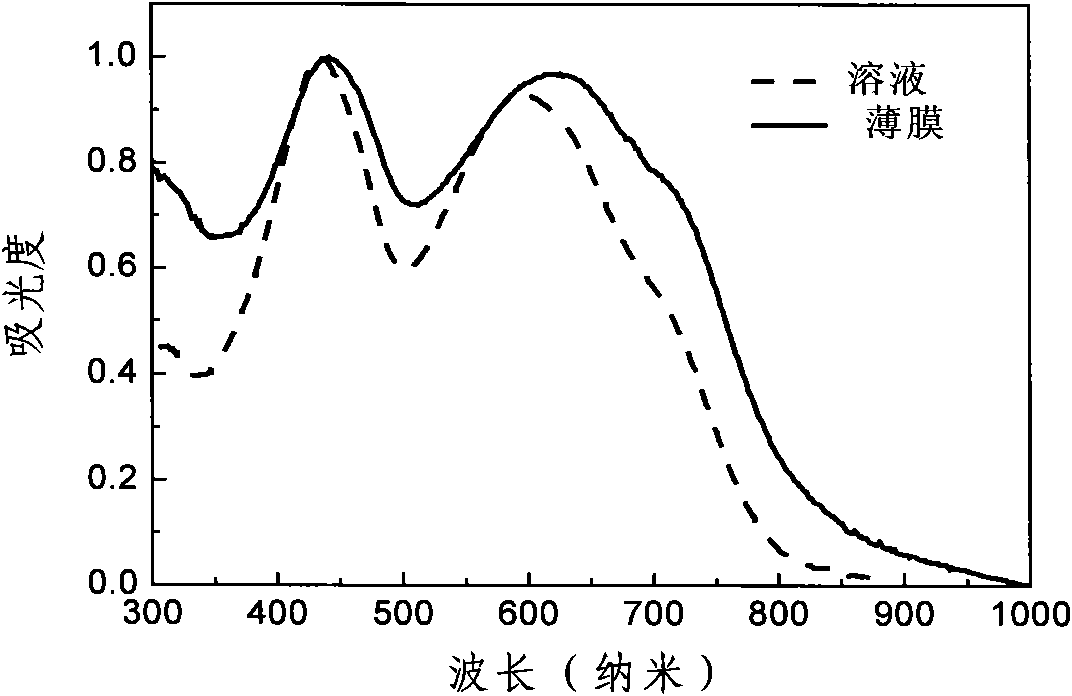
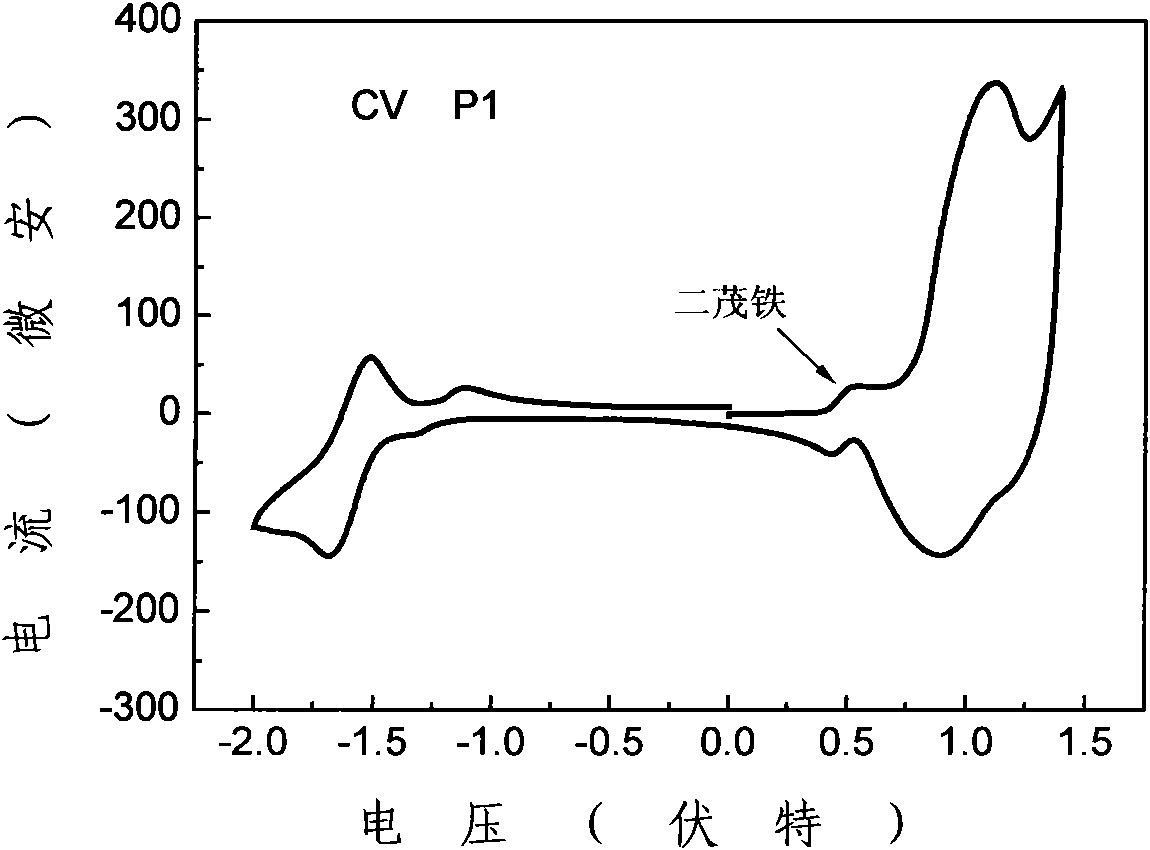
[0049] The synthetic route of polymer P1 is as follows figure 1 shown.
[0050] (1) 2,6-bis(3-dodecylthiophene)-trithiophene
[0051] Add 2-bromo-3-dodecylthiophene (1.25mmol, 664mg) and 2,6-bis(tributyltin)trithiophene (0.41mmol, 320mg) into a 50ml three-necked flask, and deoxygenate with nitrogen for 30 minutes , inject 10ml of dry toluene under the condition of isolated air, and add the catalyst Pd(PPh 3 ) 4 (Tetrakistriphenylphosphopalladium) (26 μmol, 30 mg), heated to 110° C., and reacted overnight with stirring. Cool to room temperature, add potassium fluoride aqueous solution 10ml (8g KF) and stir for 2.5 hours to precipitate and remove organic tin impurities. The reaction solution was extracted with chloroform, and the organic phase was collected and washed twice, dried over anhydrous magnesium sulfate, and purified by column chromatography (silica gel column, petroleum ether rinse) to obtain an orange solid (228mg, 79 %). 1 H NMR (400MHz, CDCl 3 ): δ7.29(s, 2H...
Embodiment 2
[0057] The synthetic route of polymer P2 is as follows Figure 6 shown.
[0058] (1) 2,6-Dibromo-3,5-Didecyltrithiophene
[0059] Add 3,5-didecyltrithiophene (0.42 g, 0.88 mmol), 10 ml of chloroform, and 10 ml of glacial acetic acid into a 100 ml single-necked bottle. The one-mouth bottle was wrapped with tin foil and cooled to 0°C. NBS (N-bromosuccinimide) (410mg, 2.30mmol) was dissolved in 3ml of dimethylformamide (DMF), then added dropwise to the one-necked bottle, stirred, and reacted for 1 hour to rise to Stir at room temperature for 4 hours. The yellow mixture was added to 200ml (2M) iced NaOH solution and stirred for 10 minutes. The organic phase was then extracted with 200ml×2 of dichloromethane, collected and dried over anhydrous magnesium sulfate. After filtration and spin-drying, it was washed with petroleum ether and separated through a silica gel column, and the solvent was removed to obtain a pale yellow solid (385 mg, 69%). 1 HNMR (400MHz, CDCl 3 ): δ2.73...
Embodiment 3
[0063] The synthetic route of polymer P3 is as follows Figure 11 shown.
[0064] Poly{[4,4-bis(decyl)dithienocyclopentadiene]-alternate-(2,1,3-benzothiadiazole)} (P3)
[0065] Add 2,7-dibromo-4,4-didecyldithienocyclopentadiene (2,7-dibromo-4,4-didecyldithiophene to a 25 ml three-neck round bottom flask Pentacyclopentadiene was synthesized according to the following literature: M.Zhang, H.N.Tsao, W.Pisula, C.Yang, A.K.Mishra, K.Müllen, "Field-effecttransistors based on a benzothiadiazole-cyclopentadithiophene copolymer", J.Am. Chem.Soc., 2007,129,3472) (123mg, 0.2mmol) and double borate substituted benzothiadiazole (78mg, 0.2mmol), then add toluene (2ml) and potassium carbonate aqueous solution (2ml, 550mg , 4 mmol), and deoxygenated by nitrogen for 30 minutes. Under the protection of nitrogen, the catalyst Pd(PPh 3 ) 4 (10 μmol, 12 mg), heated to 100°C. The reaction was stirred at 100°C for 3 days. Cool to room temperature, extract the organic phase with 100ml×2 chloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com