Method for synthesizing adamantane acid ester
A technology of adamantanoic acid ester and a synthesis method, which is applied in the field of adamantanoic acid ester synthesis, can solve problems such as being unsuitable for industrial production, difficult to purify by reaction, and achieve a simple and easy separation method, high yield, and easy experimental conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
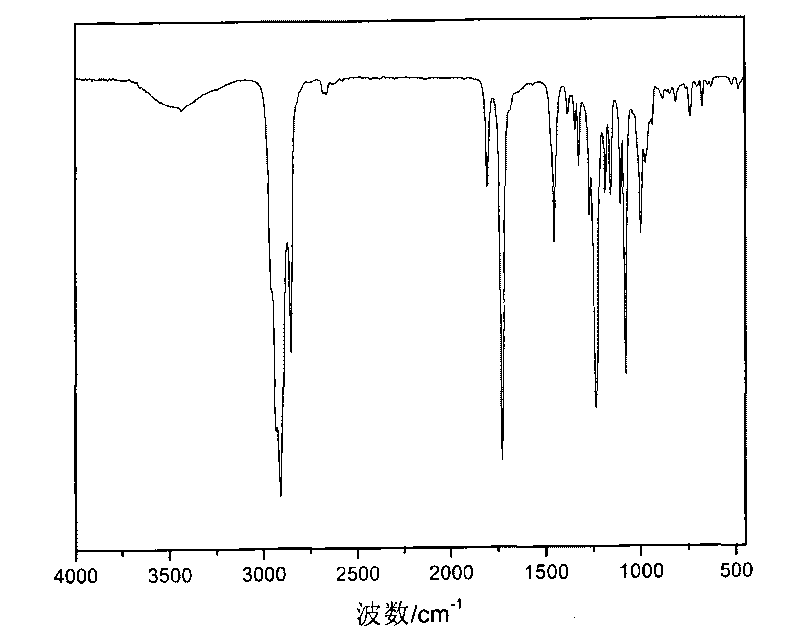
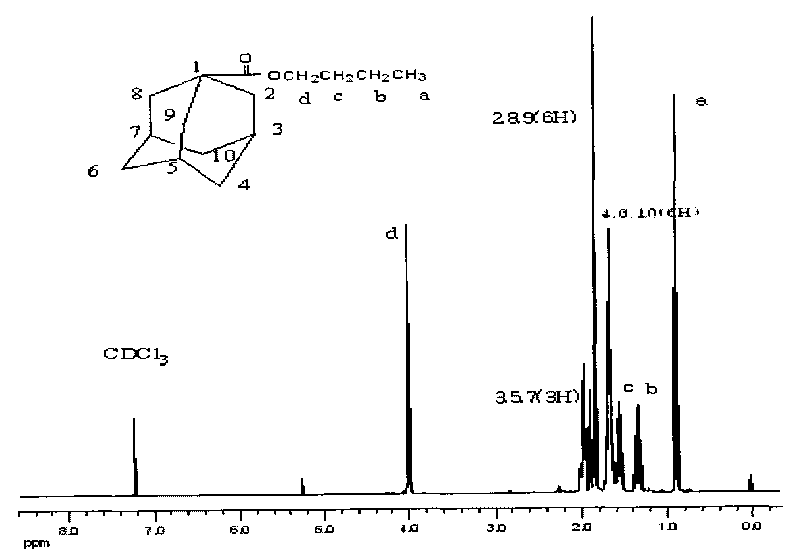
[0022] Dry adamantanecarboxylic acid in a vacuum drying oven at 85°C for 3h, take 5g (27.2mmol) of adamantanecarboxylic acid and 10ml of dichloromethane into a 50ml round-bottomed flask, and drop slowly at a rate of 0.5ml / min with a constant pressure dropping funnel Add a mixture of oxalyl chloride 4ml and dichloromethane 10ml, and react at 5°C for 6h to obtain a brown-black liquid. Unreacted oxalyl chloride was removed by rotary evaporation to obtain adamantanecarbonyl chloride.
[0023] Add dropwise a mixture of n-butanol 2ml (21.7mmol), dichloromethane 4ml and pyridine 2ml to the above product, react in an ice bath at 0°C (reaction temperature is -20-30°C) for 2h, and filter to remove Pyridinium salt, wash the filtrate with 1mol / L hydrochloric acid, wash with water, separate the liquid, wash with alkali again, and separate the oil phase liquid. An appropriate amount of calcium chloride was added to dry the product, filtered, and the remaining dichloromethane was removed by...
Embodiment 2
[0026] Dry adamantanecarboxylic acid in a vacuum oven at 85°C for 5h, take 5g (27.2mmol) of adamantanecarboxylic acid and 10ml of dichloromethane in a 50ml round-bottomed flask, and drop slowly at a rate of 0.5ml / min with a constant pressure dropping funnel Add excess 6ml of oxalyl chloride and 10ml of dichloromethane to react at 20°C for 1 hour to obtain a brownish-black liquid. Unreacted oxalyl chloride was removed by rotary evaporation to obtain adamantanecarbonyl chloride.
[0027] Add dropwise a mixture of 4ml of lauryl alcohol (18.1mmol), 6ml of dichloromethane and 4ml of freshly distilled pyridine to the above product, react at -10°C for 8h, remove the pyridinium salt by filtration, wash the filtrate with 2mol / L hydrochloric acid, and wash with water , liquid separation, and then alkali washing to separate the oil phase liquid. An appropriate amount of calcium chloride was added to dry the product, filtered, and the remaining dichloromethane was removed by rotary evapo...
Embodiment 3
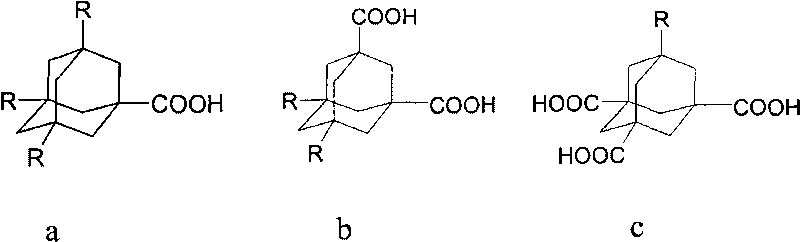
[0029] 1,3-adamantanedicarboxylic acid was dried in a vacuum oven at 90°C for 5h, 5g (22.3mmol) of 1,3-adamantanedicarboxylic acid and 10ml chloroform were placed in a 50ml round bottom flask, and the Slowly add a mixture of excess oxalyl chloride 12ml and dichloromethane 10ml dropwise into the funnel at a rate of 1.5ml / min, and react at -10°C for 3h to obtain a brownish-black liquid. Unreacted oxalyl chloride was removed by rotary evaporation to obtain adamantanoyl chloride.
[0030] Add dropwise a mixture of n-octanol 13ml (83.3mmol), dichloromethane 6ml and freshly distilled pyridine 4ml to the above product, react in an ice-bath environment at 0°C for 8h, filter to remove the pyridine salt, and wash with 1mol / L hydrochloric acid The filtrate is washed with water, separated, and then washed with alkali to separate the oil phase liquid. An appropriate amount of calcium chloride was added to dry the product, filtered, and the remaining dichloromethane was removed by rotary e...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap