Kit for quantitatively detecting vibrio parahaemolyticus in food and clinic sample
A technology for quantitative detection of hemolytic vibrio, applied in the field of kits, can solve the problems of delayed diagnosis and treatment, high fatality rate, and long time consumption, and achieve the effect of good sensitivity and rapid early diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the development of Vibrio parahaemolyticus detection reagent
[0056] 1. Design of primers and probes: through sequence comparison and analysis of all the existing Vibrio parahaemolyticus nucleic acid sequences in the Genbank database and the nucleic acid sequences reported in published literature at home and abroad, to identify pathogenic bacteria with Vibrio parahaemolyticus The relevant main virulence factor exotoxin gene is the amplified target site, selects a highly conserved segment without secondary structure, and uses software and manual design of multiple pairs of primers and probes according to the basic principles of primer and probe design.
[0057] 2. Selection of clinical samples: According to the relevant literature reports at home and abroad, the samples for testing can be selected from various food samples, such as seafood or salt pickled products. The common ones are crabs, squid, jellyfish, fish, yellow mud snails, etc. , followed by egg...
Embodiment 2
[0075] Embodiment 2: Vibrio parahaemolyticus detection kit and its application
[0076]1. Prepare a kit including the following components: 2 tubes of DNA extraction solution (500ul / tube), 20 tubes of PCR amplification reaction solution (20ul / tube), 1 tube of negative quality control (100ul / tube), positive quality control 1 tube of product (50ul / tube), 4 tubes of quantitative standard (50ul / tube).
[0077] 2. Specimen collection, transportation and storage
[0078] (1) Specimen collection: including various food specimens, such as blood, urine, sputum, pus, and puncture fluid, etc., as well as various clinical specimens from hospitals, such as vomit, blood, feces, etc., according to PCR Sampling is required to operate; operate in accordance with the national microbiological inspection standards for cosmetics. Sealed for inspection.
[0079] (2) Specimen storage and delivery: The specimens can be used for testing immediately, or can be stored at -20°C for testing, and the st...
Embodiment 3
[0088] Example 3: Quantitative detection of clinical samples using Vibrio parahaemolyticus detection kit
[0089] The clinical samples come from the semi-solid culture of Nanjing Children's Hospital, and 50 ul of the upper layer of the culture is directly taken as the test object. Specimen processing, DNA extraction, PCR reaction and result analysis are carried out with reference to Example 2.
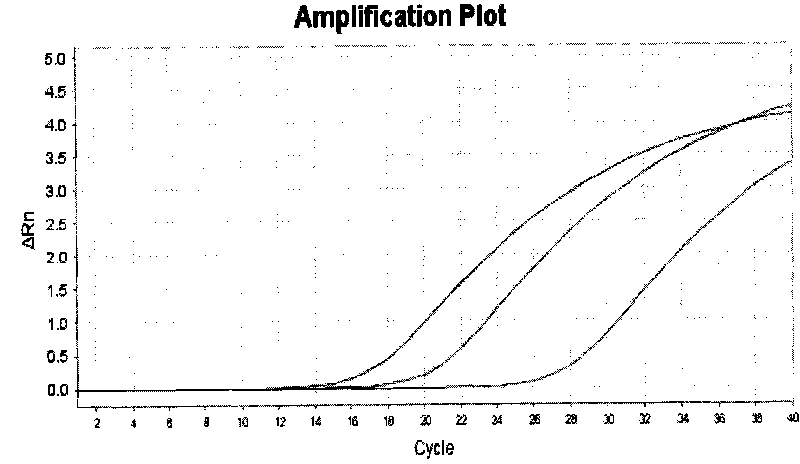
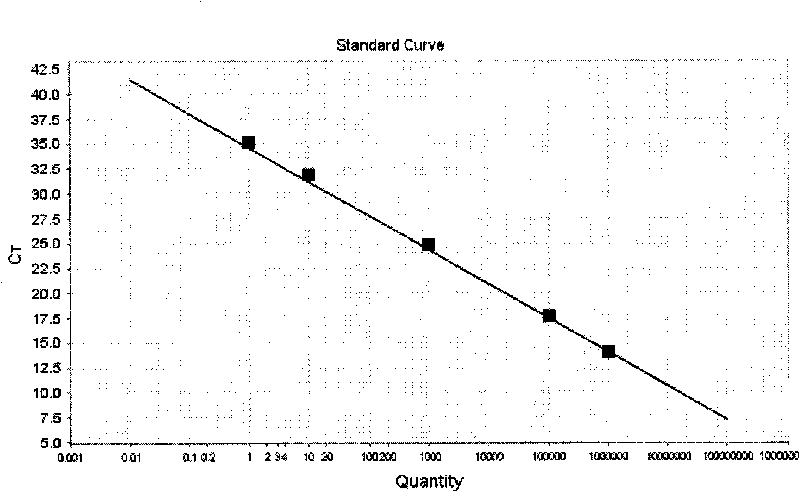
[0090] After the PCR reaction, adjust the analysis parameters according to the amplification curve to make the standard curve under the standard curve (Std curve) window reach the best (ie, the absolute value of the correlation value > 0.97), and then analyze the clinical samples. The test results of clinical samples are attached Figure 5 Shown: The Ct values of the amplification curves of the six clinically positive samples are 23.83, 24.63, 26.02, 27.05, 27.28, and 29.61, respectively. Combined with the obvious exponential growth period of the amplification curves, all of them can...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com