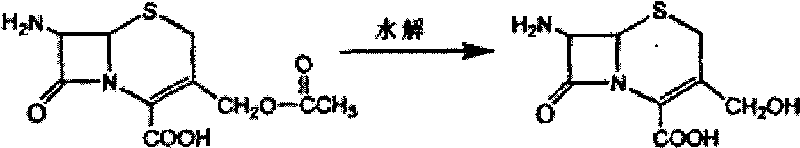Synthesis Method of cefcapene pivoxil hydrochloride
A technology of cefcapene pivoxil hydrochloride and cefcapene pivoxil, which is applied in the field of antibiotic drug synthesis, can solve the problems of high corrosion resistance of reaction vessels, poor product quality, complicated operation, etc., and achieve high implementation value, mild reaction conditions, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of 7-DACA
[0027] 266 kg of water and 10.6 kg of sodium hydroxide were stirred and dissolved, then 174 kg of acetone and 2.5 kg of boric acid were added while cooling down, the temperature was lowered to -20~-15°C, 33 kg of 7-ACA was added, and the temperature was controlled for 60 minutes to dissolve them all. After adding 29 kg of 6N hydrochloric acid dropwise, transfer it to 840 kg of water. The mixed solution is at about 13°C. Slowly add 6N hydrochloric acid to adjust the pH value to about 6.4. Continue to adjust the pH value to 3.2 under temperature control for 30 minutes. Stir at temperature control for 60 minutes, centrifuge Filter, first wash with 120 kg of water, then wash twice with 40 kg of acetone. With dehydration, drying under normal pressure below 40°C for 30-60 minutes to obtain about 20 kilograms of products with moisture below 1.0%.
Embodiment 2
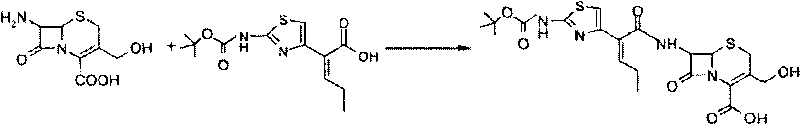
[0028] Embodiment 2: the synthesis of carbonyl tert-butyl cefcapene acid
[0029] A. The preparation of the mother nucleus: add 34.8 kg of methanol, add 15 kg of 7-DACA, add 14.5 kg of triethylamine dropwise at 0-5°C for about 10-15 minutes, and stir at this temperature for about 90-120 minutes to dissolve all . After dissolving, lower it to below 0°C for later use.
[0030] B, side chain acid preparation: add 187 kilograms of ethyl acetate, cefcapine pivoxil side chain acid ((Z)-2-(2-carbonyl tert-butoxyaminothiazol-4-yl)-2-pentane in the dry kettle enoic acid) 23.3 kg, cooled to 0°C, 10.3 kg of methanesulfonyl chloride was added dropwise at this temperature, and 9.14 kg of pyridine was added dropwise at -5~0°C. Temperature control reaction for 2 hours, 1.5 hours sampling side chain acid residual less than 3% is the reaction end point. Cool down to -10°C for dropwise addition.
[0031] Add the side chain acid chloride solution of B to the prepared hydrolyzed material solu...
Embodiment 3
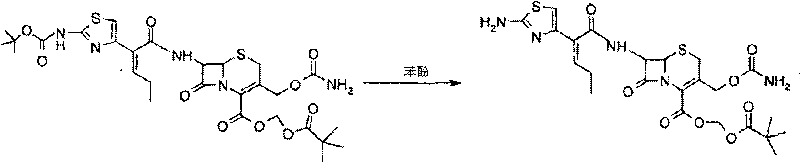
[0032] Embodiment 3: the synthesis of carbonyl tert-butoxy cefcapene pivoxil
[0033] Add 32 kg of cefcapene acid, add 200 kg of DMF to dissolve, control the temperature at 20-25°C, slowly add 1.5 kg of potassium iodide, cool down to 0-5°C, add 13.6 kg of chloromethyl pivalate dropwise in about 30 minutes, and control the temperature React for 3 hours, take a sample to detect that the residual cefcapenic acid is less than 0.5%, add 300 kg of dichloromethane, 0.5 kg of tetraheptylammonium bromide, add 400 kg of water dropwise, pay attention to cooling, the reaction exotherm is obvious, stir for 30 minutes to separate layers, The obtained aqueous phase was extracted once with 50 kg of dichloromethane, the obtained organic phase was washed and separated with 5% sodium bicarbonate, the aqueous phase was extracted with the above-mentioned dichloromethane, the combined organic phases were concentrated to dryness.
[0034] 68 kg of ethyl acetate was added to the concentrated solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com