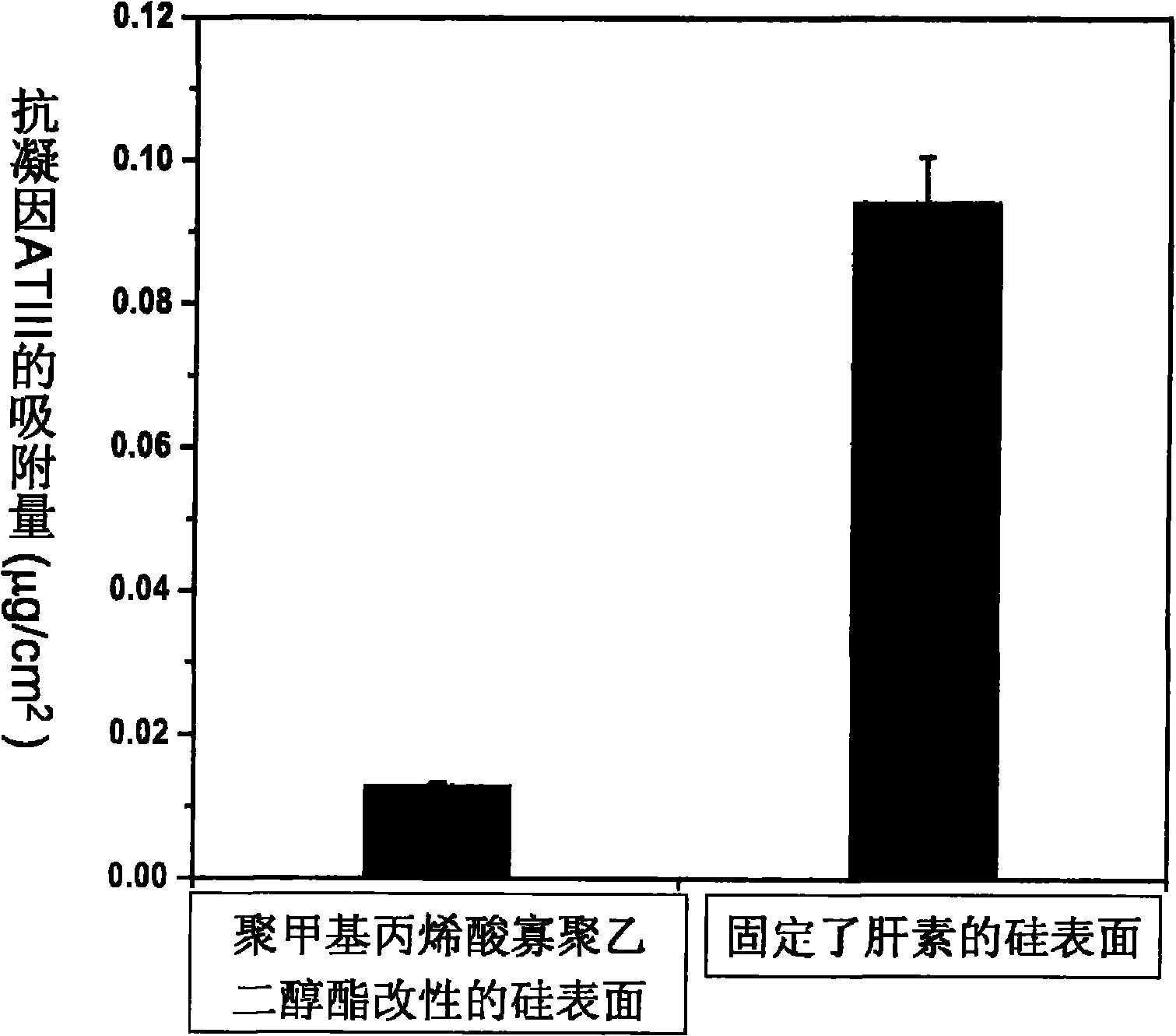
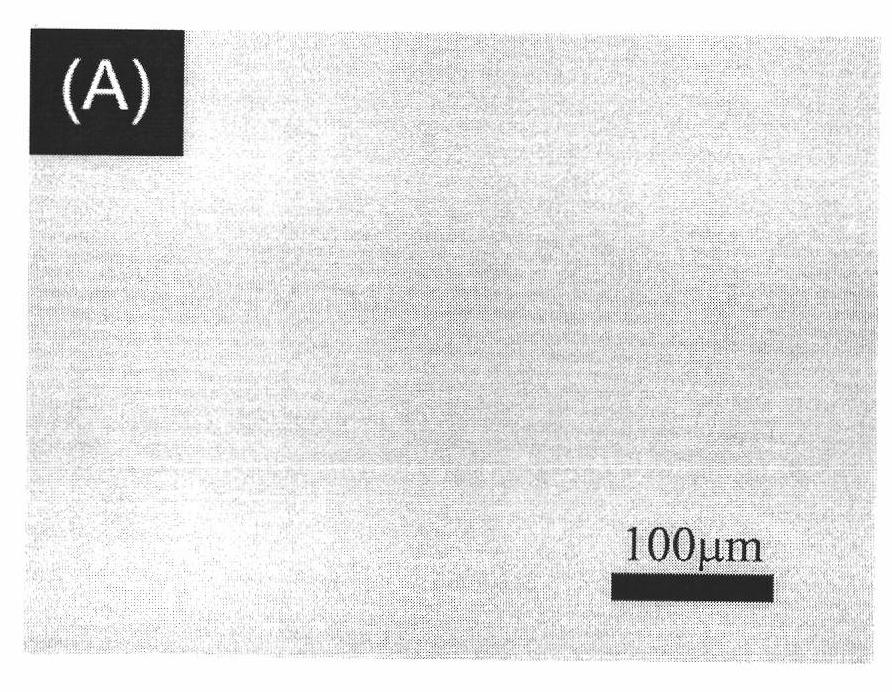
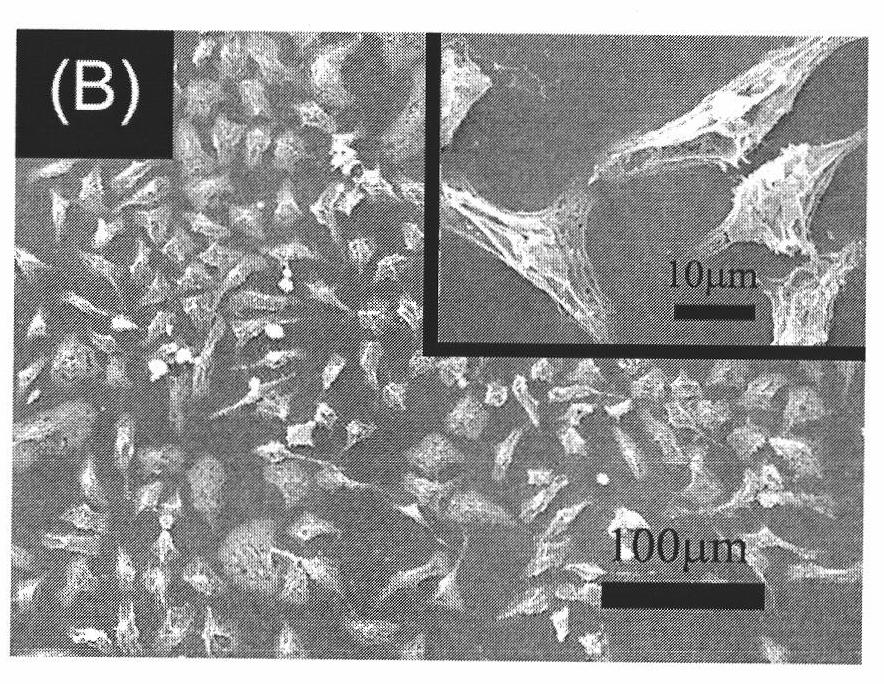
Preparation method for material with high density fixed biologically functional molecule
A technology of functional molecules and biologically active molecules, which is applied in the fields of tissue engineering, polymer materials, and biological detection, can solve the problems of low density of immobilized biomolecules and complicated reaction process, and achieve the promotion of cell adhesion and spreading, simple operation, and applicability wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 2.2 mL of methacryloyl chloride in 10 mL of chloroform was added dropwise to a solution of 2.3 g of NHS and 3.3 mL of triethylamine in 20 mL of chloroform at 0°C. After the dropwise addition was completed, the reaction was carried out at room temperature for 4 hours. The reaction solution was washed with saturated ice brine, and the organic phase was dried with anhydrous magnesium sulfate. The precipitate was filtered, the solvent was concentrated, and then recrystallized in a mixed solution of 0.8 mL ethyl acetate and 6 mL n-hexane to obtain NHSMA as a monomer.
[0036] Under a nitrogen atmosphere, 7.93 g of OEGMA, 312 mg of 2,2-bipyridine and 143 mg of cuprous bromide were dissolved in 15 mL of a mixed solution of methanol and water (4:1). The above solution was ultrasonically transferred to a silicon chip with α-bromobutyryl bromide initiator fixed on the surface for 5 minutes, reacted at room temperature for 2 hours, then stopped the reaction with copper bromide / bi...
Embodiment 2
[0039] 2.2 mL of methacryloyl chloride in 10 mL of chloroform was added dropwise to a solution of 2.3 g of NHS and 3.3 mL of triethylamine in 20 mL of chloroform at 0°C. After the dropwise addition was completed, the reaction was carried out at room temperature for 4 hours. The reaction solution was washed with saturated ice brine, and the organic phase was dried with anhydrous magnesium sulfate. The precipitate was filtered, the solvent was concentrated, and then recrystallized in a mixed solution of 0.8 mL ethyl acetate and 6 mL n-hexane to obtain NHSMA as a monomer.
[0040] Under a nitrogen atmosphere, 7.93 g of OEGMA, 312 mg of 2,2-bipyridine and 143 mg of cuprous bromide were dissolved in a mixed solution of 15 mL of methanol and water (1:1). After the above solution was ultrasonicated for 5 minutes, it was transferred to a silicon wafer with α-bromobutyryl bromide initiator fixed on the surface, reacted at room temperature for 2 hours, and the surface was cleaned with ...
Embodiment 3
[0043] 2.2 mL of methacryloyl chloride in 10 mL of chloroform was added dropwise to a solution of 2.3 g of NHS and 3.3 mL of triethylamine in 20 mL of chloroform at 0°C. After the dropwise addition was completed, the reaction was carried out at room temperature for 4 hours. The reaction solution was washed with saturated ice brine, and the organic phase was dried with anhydrous magnesium sulfate. The precipitate was filtered, the solvent was concentrated, and then recrystallized in a mixed solution of 0.8 mL ethyl acetate and 6 mL n-hexane to obtain NHSMA as a monomer.
[0044] Under a nitrogen atmosphere, 7.93 g of OEGMA, 312 mg of 2,2-bipyridine and 143 mg of cuprous bromide were dissolved in 15 mL of a mixed solution of methanol and water (2:1). The above solution was ultrasonicated for 5 minutes and then transferred to a silicon wafer with α-bromobutyryl bromide initiator fixed on the surface. After reacting for 2 hours at room temperature, the surface was cleaned with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com