Housefly cecropin-human lysozyme fusion protein, and preparation method and application thereof
A technology of housefly cecropin and human lysozyme, which is applied in the field of preparation of genetically engineered antibacterial proteins, can solve the problems of not wide antibacterial spectrum, high cost, and inconspicuous antibacterial effect, so as to improve expression efficiency and reduce the probability of tolerance , the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
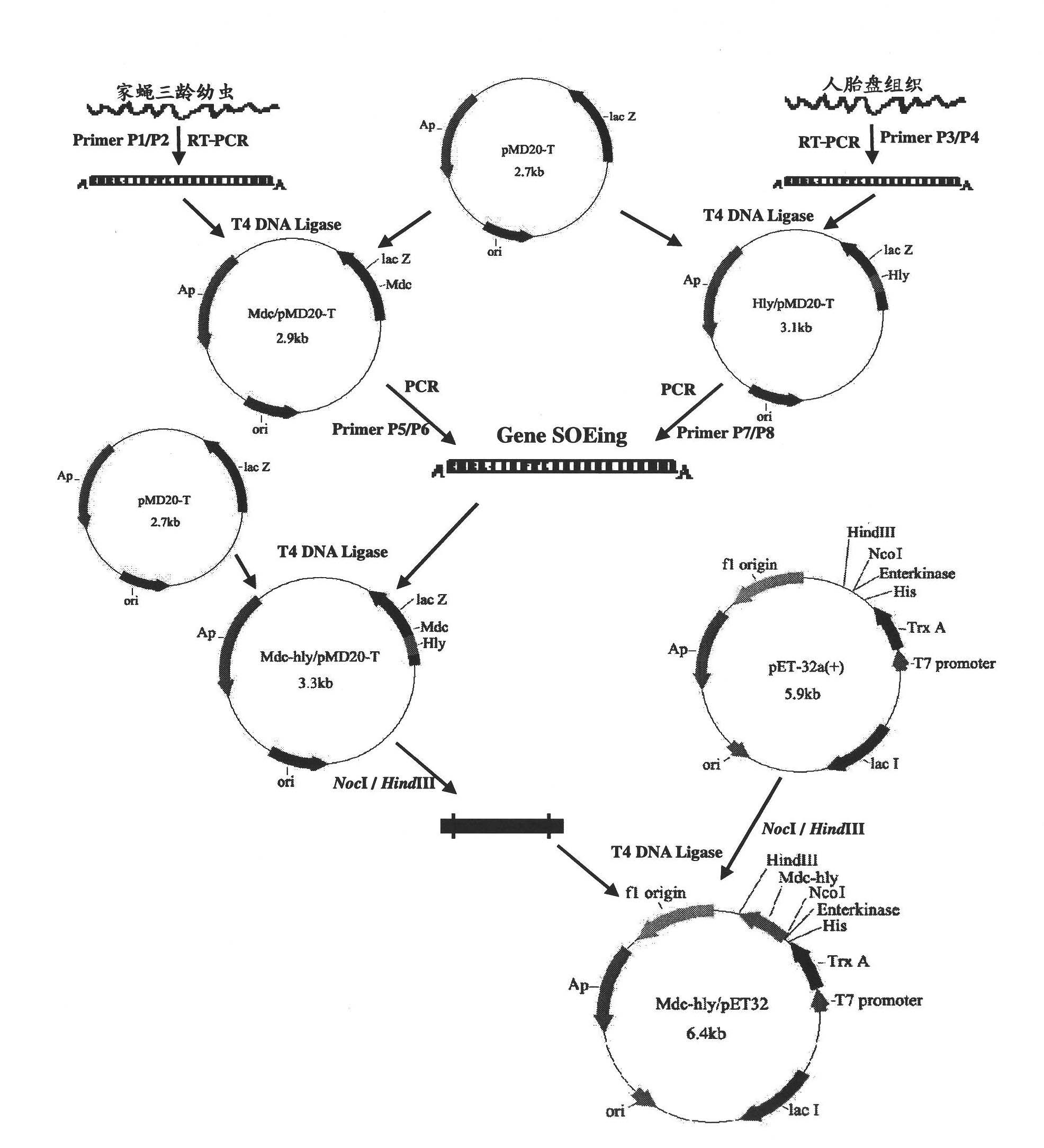
[0035] This program uses the pET expression system of Novagen to express the fusion gene Mdc-hly, the pET32a(+) plasmid is used as the expression vector, and the host cell is Escherichia coli.
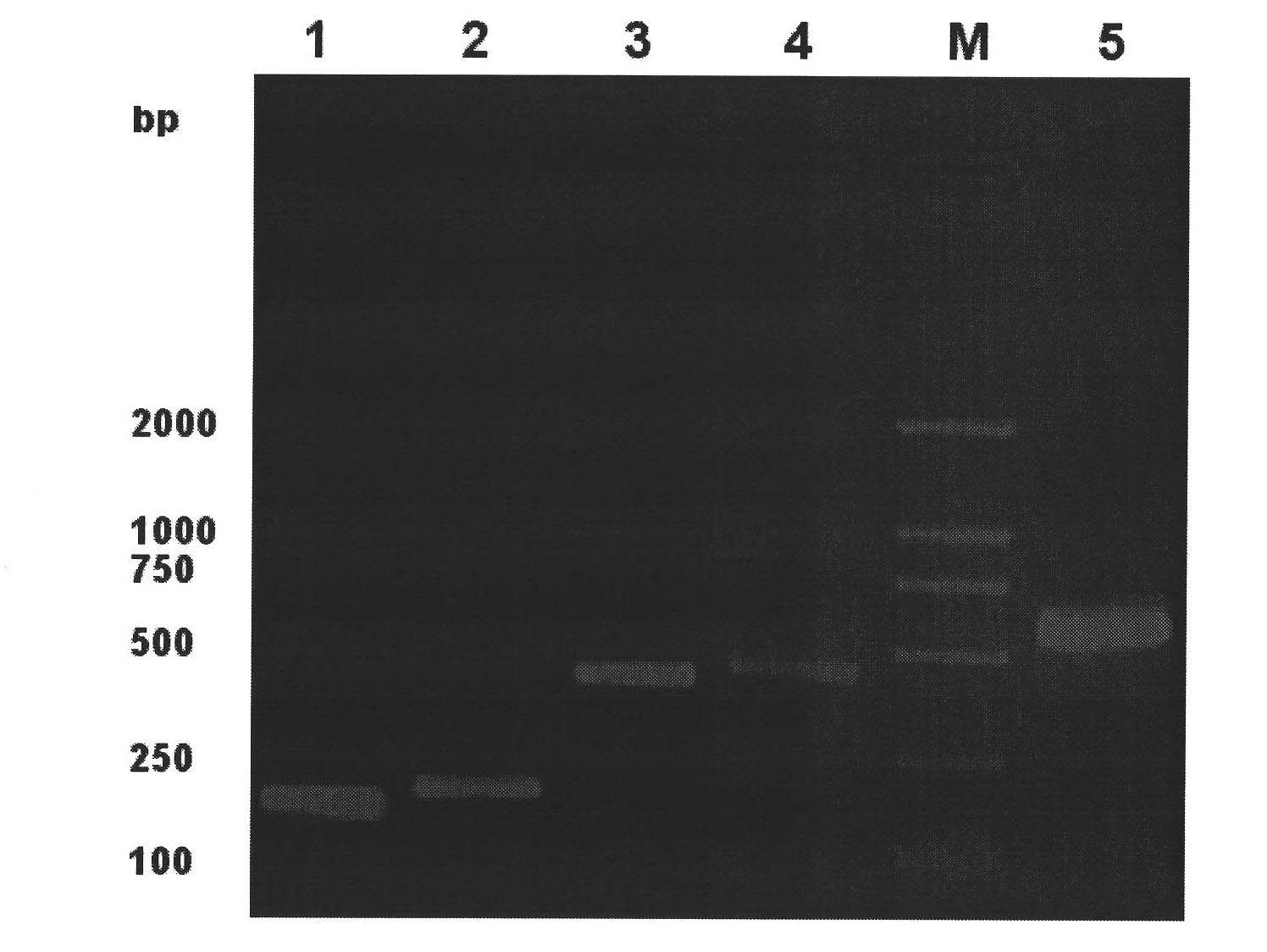
[0036] 1 Cloning of the mature peptide sequences of Musca muscae cecropin (Mdc) and human lysozyme (Hly) (see figure 1 )
[0037] According to the instructions of Trizol Reagent, total RNA was extracted from housefly third-instar larvae and human placenta respectively. According to the cDNA sequences of cecropin (Genebank accession number: EF175878) and human lysozyme (Genebank accession number: J03801) published by Genebank, Primer Premier 5.0 Biology software designed 4 specific primers, P1~P4, and the primer sequences are shown in SEQ ID NO:6~9. Primers P1 and P2 amplify the mature peptide sequence of Cecropin, wherein the upstream P1 introduces the Nco I restriction site, the downstream P2 removes the stop codon, adds an asparagine codon AAC and introduces the BamH I restriction sit...
Embodiment 2
[0099] Example 2 Bacteriostatic activity analysis of fusion protein Mdc-hly
[0100] Micrococcus aureus (S.aureus) ATCC25923, M.lysodeikticus (M.lysodeikticus) CICC23645, Bacillus subtilis (B.subtilis) ATCC6633, and Escherichia coli (E. ) ATCC25922, Pseudomonas aeruginosa (P.aeruginosa) ATCC27853, Salmonella (S.paratyphi-B) CICC 21495 MIC value. Dilute Mdc, Hly, and Mdc-hly with sterile Milli-Q water to final concentrations of 200, 100, 75, 50, 25, 12.5, 6.25, 3.121.56, 0.78, and 0.39uM, respectively. Inoculate single colonies into Mueller Hinton (referred to as M-H) medium respectively, after overnight culture, inoculate in fresh M-H medium at a ratio of 1:100, cultivate to logarithmic growth phase at 35°C, count under the microscope on a hemocytometer, and use Adjust the concentration of M-H culture medium to 2×10 6 CFU / ml (colony forming units per ml). Take a sterile 96-well plate, add 100 microliters of freshly prepared bacterial solution to each well, and then add 100 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com