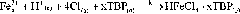Method for producing alumina through bauxite acid process
A technology of bauxite and alumina, applied in chemical instruments and methods, aluminum compounds, inorganic chemistry, etc., can solve the problems of low comprehensive utilization rate of resources, large amount of dissolved red mud, complicated process, etc., and increase ore processing capacity , high resource utilization and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
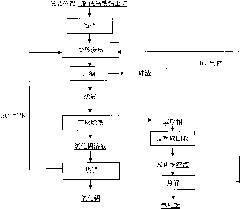
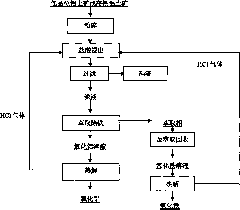
[0034] The aluminum-silicon ratio in the bauxite used is 1.5. The bauxite is crushed and finely ground until the particle size is less than 150 μm, and then placed in a closed container and leached with hydrochloric acid. During the leaching process, hydrogen chloride gas is introduced into the device. The amount of hydrogen chloride gas added It is 0.6t / t bauxite; the weight concentration of hydrochloric acid used is 5%, the liquid-solid ratio of hydrochloric acid and bauxite is 20:1L / kg, the temperature of the material during leaching is 80°C, and the leaching time is 240min. The leaching rate of aluminum element was 91.58%, and the leaching rate of iron element was 95.42%. The ore slurry was separated by solid-liquid to obtain leaching liquid and leaching slag, and the mass content of silicon dioxide in the leaching slag was 88.13%.
[0035] Use tributyl phosphate as extractant and sulfonated kerosene as diluent to separate the iron and aluminum elements in the leaching solu...
Embodiment 2
[0040]The aluminum-silicon ratio in the bauxite used is 2.5. The bauxite is crushed and finely ground to a particle size of less than 150 μm, then placed in a closed container and leached with hydrochloric acid. During the leaching process, hydrogen chloride gas is introduced into the device. The amount of hydrogen chloride gas added It is 1.0t / t bauxite; the weight concentration of hydrochloric acid used is 20%, the liquid-solid ratio of hydrochloric acid and bauxite is 2:1L / kg, the temperature of the material during leaching is 110°C, and the leaching time is 60min. The leaching rate of aluminum element in the ore was 93.15%, and the leaching rate of iron element was 93.56%. The leachate and leaching slag were obtained through solid-liquid separation of the pulp, and the mass content of silicon dioxide in the leaching slag was 90.75%.
[0041] Use tributyl phosphate as extractant and sulfonated kerosene as diluent to separate iron and aluminum elements in the leaching solutio...
Embodiment 3
[0047] The aluminum-silicon ratio in the bauxite used is 5. The bauxite is crushed and finely ground to a particle size of less than 150 μm, then placed in a closed container and leached with hydrochloric acid. During the leaching process, hydrogen chloride gas is continuously injected into the device for injection. The amount of hydrogen chloride gas added is 0.8t / t bauxite; the initial weight concentration of hydrochloric acid is 20%, the liquid-solid ratio of hydrochloric acid and bauxite is 10:1L / kg, the temperature of the material during leaching is 170°C, and the leaching time is 30min . The leaching rate of aluminum element in the ore was 93.15%, and the leaching rate of iron element was 96.53%. The leachate and leaching slag were obtained through solid-liquid separation of the ore slurry, and the mass content of silicon dioxide in the leaching slag was 92.46%.
[0048] Use tributyl phosphate as extractant and sulfonated kerosene as diluent to separate iron and aluminum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com