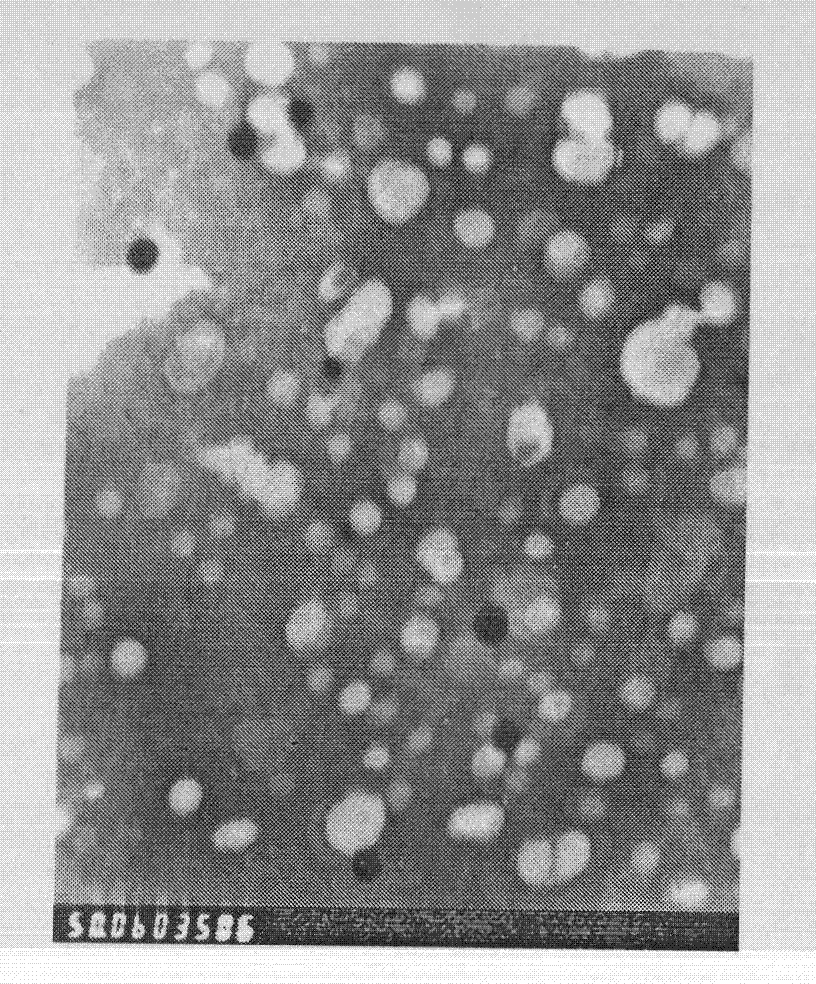
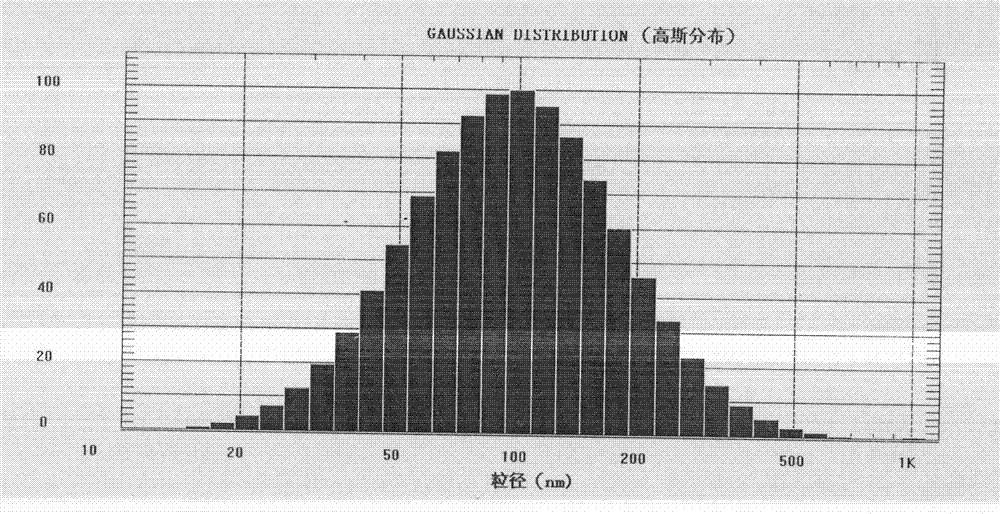
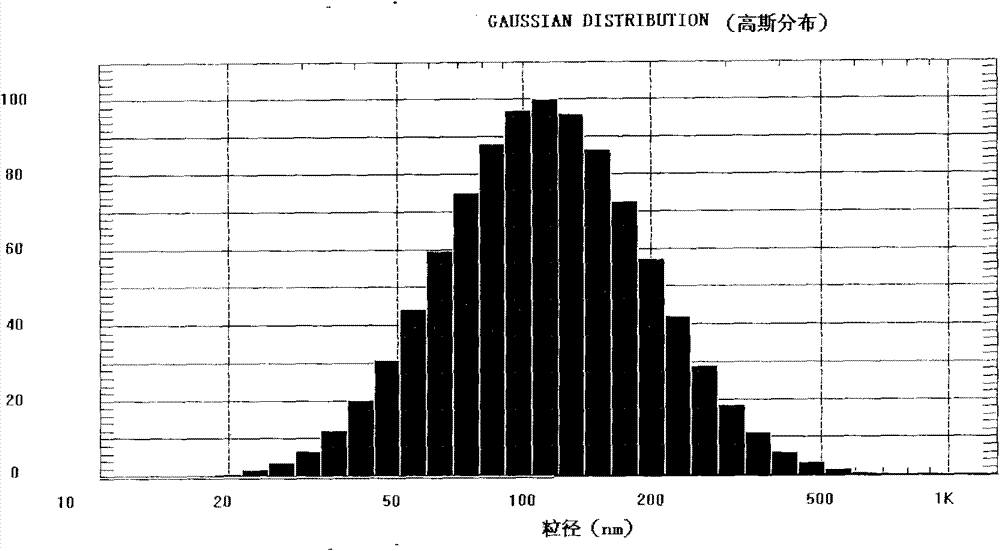
Novel long-circulating liposome composition and preparation method thereof
A long-circulation liposome and composition technology, which is applied in the directions of liposome delivery, drug combination, and pharmaceutical formulation, can solve the problems of high production cost, large mass ratio, and increased production cost of long-circulation liposomes, achieving The effect of low charge, stable quality, and obvious long cycle characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The preparation of embodiment 1 novel paclitaxel long circulation liposome
[0081] Paclitaxel 0.60g Hydrogenated Soy Lecithin 16g
[0082] Cholesterol 2.5g Vitamin E succinate 0.1g
[0083] Vitamin E polyethylene glycol succinate (TPGS 2000) 2.5g
[0084] Vitamin E 0.05g
[0085] Sucrose 20g
[0086] Water for injection 200ml
[0087] Weigh respectively paclitaxel, hydrogenated soybean lecithin, cholesterol, vitamin E succinate (VES), vitamin E polyethylene glycol succinate (TPGS 2000), vitamin E, add 100ml absolute ethanol, after dissolving, place Rotary evaporator, under the condition of nitrogen flow protection, 50 ℃ water bath constant temperature, drain the solvent, prepare a film, place it in a vacuum desiccator, and fully remove the residual solvent. Add sucrose solution dissolved in water for injection, keep the temperature at 55°C, stir with magnetic force for 30 minutes, disperse evenly in a water bath, homogenize with a high-pressure homogenizer, filter...
Embodiment 2
[0091] Embodiment 2: Preparation of novel paclitaxel long-circulating liposome
[0092] Paclitaxel 0.15g Hydrogenated Soy Lecithin 16g
[0093] Cholesterol 4.5g Vitamin E succinate 0.5g
[0094] Vitamin E Polyethylene Glycol Succinate (TPGS 1000) 2.5g
[0095] Vitamin E 0.08g
[0096] Trehalose 20g
[0097] Water for injection 200ml
[0098] Weigh paclitaxel, hydrogenated soybean lecithin, cholesterol, vitamin E succinate (VES), vitamin E polyethylene glycol succinate (TPGS 1000) and vitamin E respectively, add 100ml of absolute ethanol, after dissolving, place Rotary evaporator, under the condition of nitrogen flow protection, 50 ℃ water bath constant temperature, drain the solvent, prepare a film, place it in a vacuum desiccator, and fully remove the residual solvent. Add sucrose solution dissolved in water for injection, keep the temperature at 55°C, stir with magnetic force for 30 minutes, disperse evenly in a water bath ultrasonically, extrude with an extruder with a...
Embodiment 3
[0100] Embodiment 3: Preparation of novel paclitaxel long-circulating liposome
[0101] Paclitaxel 0.90g Hydrogenated Soy Lecithin 16g
[0102] Cholesterol 1.25g Vitamin E succinate 1.0g
[0103] Vitamin E Polyethylene Glycol Succinate (TPGS 500) 2.0g
[0104] Vitamin E 0.1g
[0105] Lactose 15g
[0106] Water for injection 200ml
[0107] Weigh the prescribed amount of paclitaxel, hydrogenated soybean lecithin, cholesterol, vitamin E succinate, vitamin E polyethylene glycol succinate (TPGS 2000), and vitamin E, add 100ml of absolute ethanol, dissolve, and place in a rotary evaporator , under the condition of nitrogen flow protection, keep the temperature in a water bath at 50°C, drain the solvent, prepare a film, place it in a vacuum desiccator, and fully remove the residual solvent. Add sucrose solution dissolved in water for injection, keep the temperature at 55°C, stir with magnetic force for 30 minutes, disperse evenly in a water bath, homogenize with a high-pressure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com