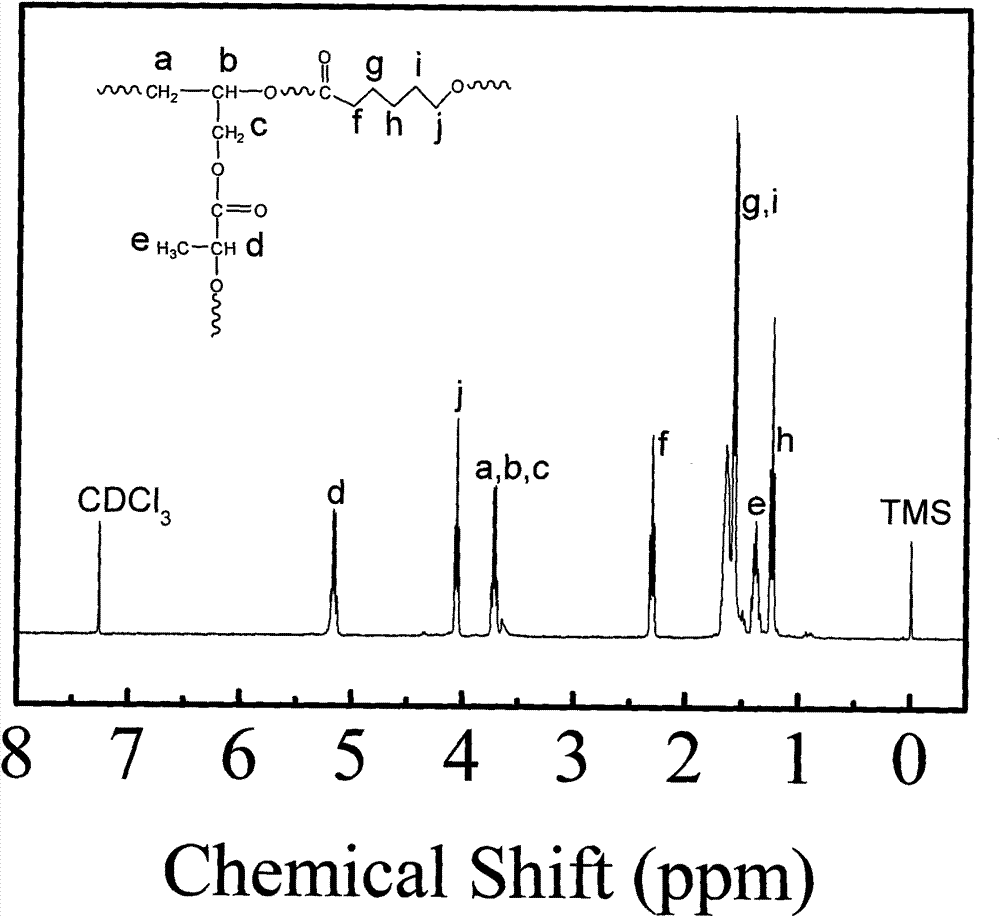
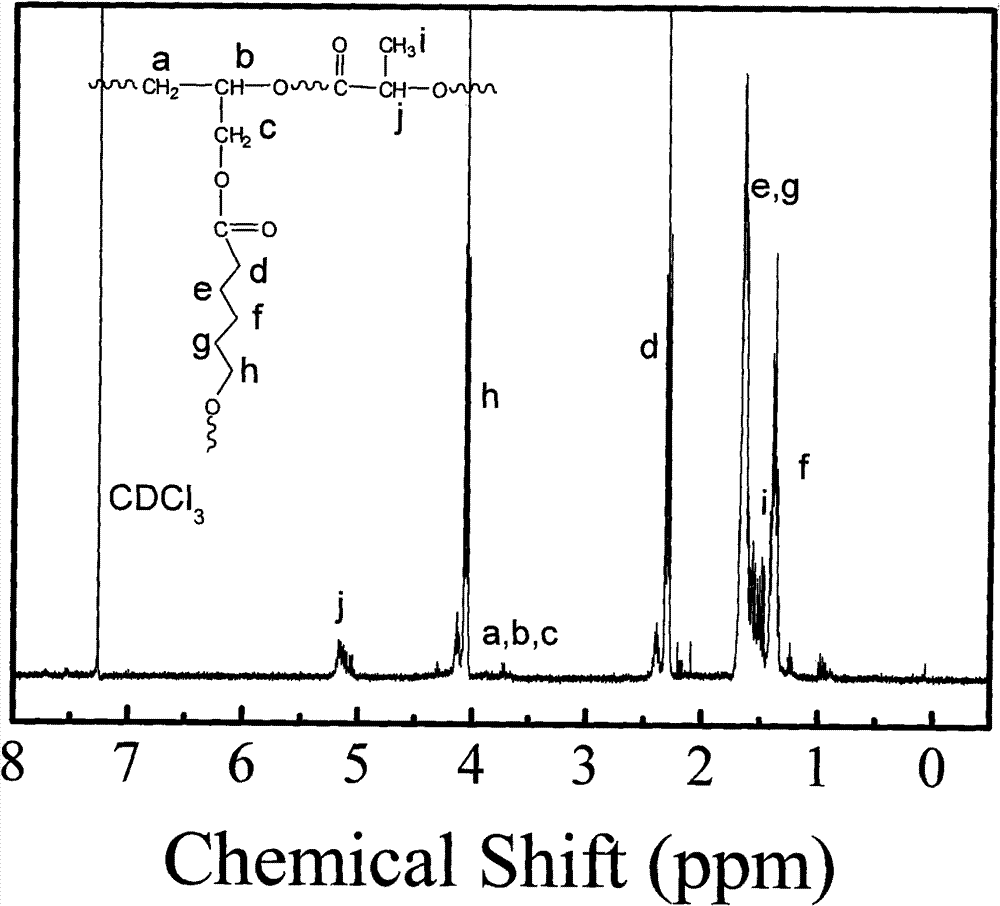
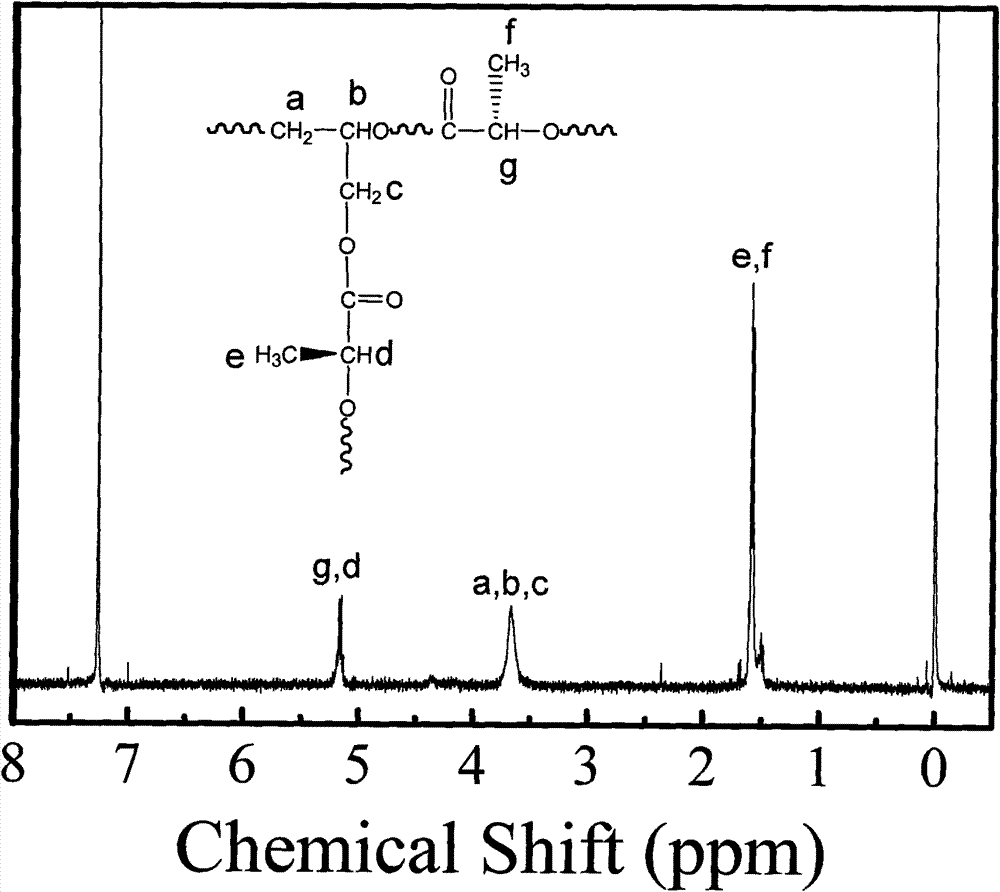
Biodegradable high-polymer additive, preparation method and application thereof
A biodegradation and additive technology, applied in the field of polymer materials, can solve problems such as no research reports, and achieve the effects of improving mechanical properties, good compatibilization effect, and reducing melt viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The specific steps and conditions for preparing the biodegradable additive with branched structure provided by the invention are as follows:
[0048] 1) preparation of macromolecular initiator containing side hydroxyl
[0049] Dissolve the protective group-containing glycidyl alcohol in tetrahydrofuran in a dry reaction vessel, heat to 30-70°C, and the reaction time is less than 72h. Until the reaction is complete, under the protection of argon, the cyclic ester mono Add the tetrahydrofuran solution of the solid body into the reaction system, react at 0-70°C for <24h, remove the organic solvent, and obtain a block copolymer containing hydroxyl protecting groups. Then, dissolve the block copolymer in a mixed solvent of organic solvent / water, and react under acidic conditions at 0-50°C for <48h to remove the ethyl ethoxy group from the side chain of the copolymer, A macromolecular initiator containing side hydroxyl groups is obtained.
[0050] 2) Preparation of additive...
Embodiment 1
[0057] Embodiment 1, preparation PCL-b-PG-g-PLLA
[0058] Add 1g of ethoxyethyl glycidyl ether into a dry reaction vessel, add 0.5mL potassium tert-butoxide tetrahydrofuran solution (1mol / L) and 3mL tetrahydrofuran at the same time, react at 60°C for 24h; At room temperature, 5 mL of caprolactone monomer tetrahydrofuran solution (0.333 g / mL) was slowly added dropwise to the reaction vessel, and reacted for 4 h at 40 ° C; the reaction mixture was poured into ether to precipitate and wash, and after drying, poly(ε -caprolactone-b-ethoxyethyl glycidyl ether) (PCL-b-PEEGE). Dissolve 3g of poly(ε-caprolactone-b-ethoxyethyl glycidyl ether) in 15ml of acetone, then add the aqueous solution of oxalic acid into the polymer solution, react at room temperature for 16h, then add calcium hydroxide, The molar ratio of hydroxyl / oxalic acid / calcium hydroxide in the polymer is 1 / 0.5 / 1. After reacting for 1 hour at room temperature, the precipitate in the solution is removed by centrifugation,...
Embodiment 2
[0060] Embodiment 2, preparation PCL-b-PG-g-PLLA
[0061] Add 1g of ethoxyethyl glycidyl ether into a dry reaction vessel, add 6.8uL potassium tert-butoxide tetrahydrofuran solution (1mol / L) and 1mL tetrahydrofuran at the same time, and react at 20°C for 168h; under the protection of argon At room temperature, slowly drop 0.75mL caprolactone monomer tetrahydrofuran solution (0.5g / mL) into the reaction vessel, and react at 0°C for 6h; pour the reaction mixture into diethyl ether for precipitation and washing, and obtain poly( ε-caprolactone-b-ethoxyethyl glycidyl ether) (PCL-b-PEEGE). Dissolve 1g of poly(ε-caprolactone-b-ethoxyethyl glycidyl ether) in 15ml of acetone, then add the aqueous solution of oxalic acid into the polymer solution, react at room temperature for 16h, then add calcium hydroxide, The molar ratio of hydroxyl / oxalic acid / calcium hydroxide in the polymer is 1 / 0.5 / 1. After reacting for 1 hour at room temperature, the precipitate in the solution is removed by c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com