Fusion protein HABP-mKate and preparation method and application thereof
A technology of fusion protein and protein, applied in the field of genetic engineering, can solve the problems of inability to produce large-scale and high extraction cost, and achieve the effects of low cost, simple preparation, sensitive detection and specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Preparation of embodiment one fusion protein HABP-mKate
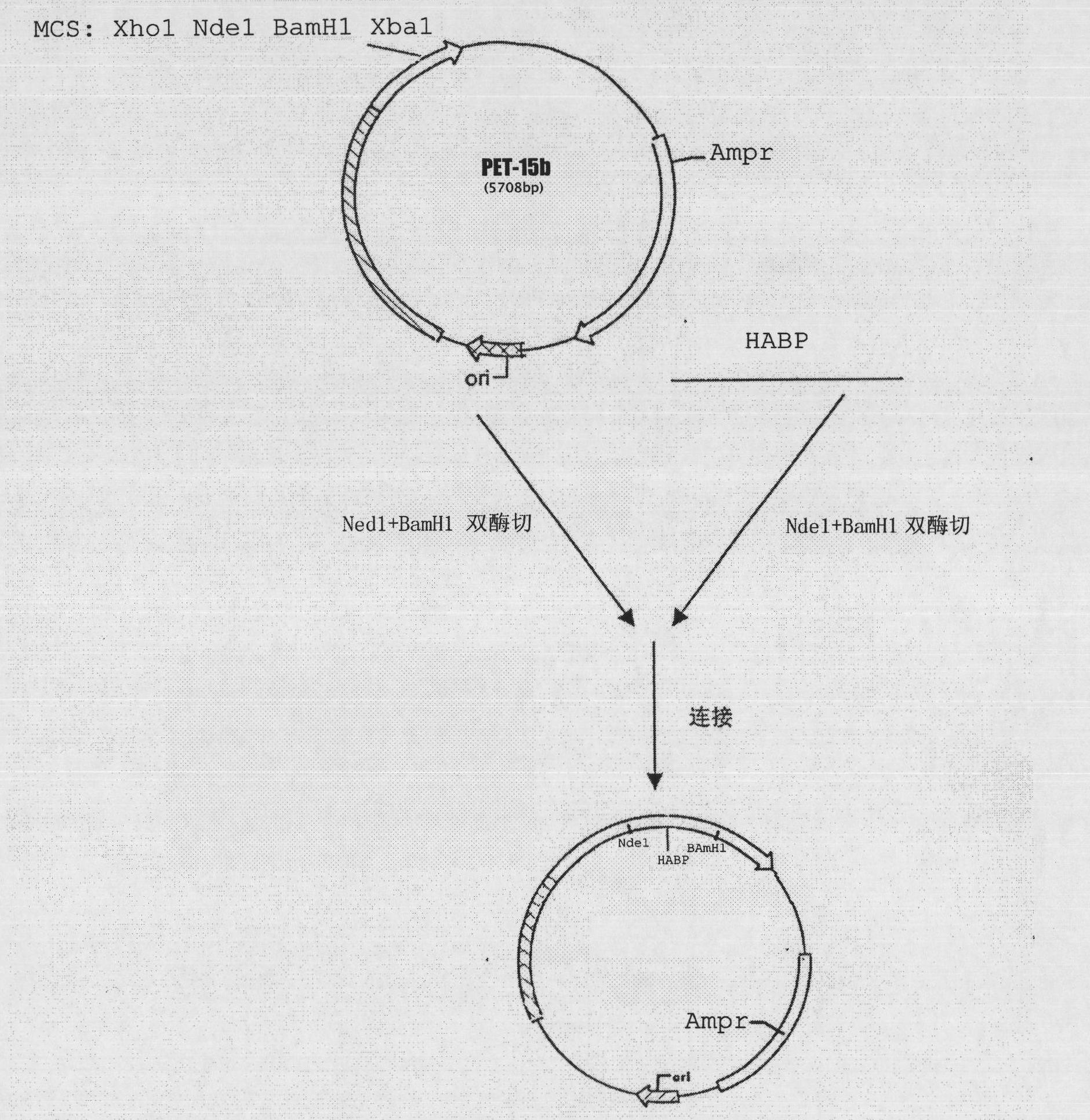
[0023] 1. Gene optimization and synthesis
[0024] Link the amino acid sequences of HABP and mKate, and add a linker in the middle (see figure 2 ), and then input the linked HABP-mKate amino acid sequence (SEQ ID NO.2) into the IDT gene optimization software (http: / / www.idtdna.com / ) for codon bias analysis (Escherichia coli) , select commonly used codons in the Escherichia coli expression system, optimize the amino acid sequence, and obtain the gene sequence (SEQ ID NO.1) corresponding to the amino acid sequence of HABP-mKate. The gene sequence is input into gene2oligo (http: / / berry.engin.umich.edu / gene2oligo / ) to obtain the corresponding Oligo, and these Oligo are synthesized in vitro by overlapping PCR method. HABP-mKate fusion gene, in the HABP-mKate fusion gene Select and design PCR primers on both sides, and synthesize two by chemical synthesis, respectively:
[0025]P1: TTCATATGGGTGTTTATCACCGCG (5-primer...
Embodiment 2
[0034] 1. Analysis of the binding ability of fusion protein HABP-mKate to HA
[0035] In order to detect the specific binding ability of the fusion protein HABP-mKate to HA, we coated the HA protein (Sigma, St Louis, MO) at a concentration of 1 mg / ml on a microwell plate overnight at room temperature, and then used After washing three times with PBS, they were blocked with 0.5% casein for 2 hours at room temperature. At this time, we added HABP-mKate (0.05-10ug / ml) prepared in Example 1, HABP-mKate and free low molecular weight HA (HA24) (from sigma company) and blank to the above holes respectively. For comparison, the result is Figure 5 , only HABP-mKate has an OD650 as high as 0.45, but when there is HA24 or the blank control, the OD650 only reaches about 0.1, indicating that the fusion protein HABP-mKate has a specific binding ability to HA.
[0036] 2. Analysis of the binding ability of fusion protein HABP-mKate to 4T1 cells specifically expressing HA
[0037] In orde...
Embodiment 3
[0045] Fluorescent Stability Test of Embodiment 3 HABP-mKate
[0046] In order to judge the feasibility of HABP-mKate as a reagent for detecting HA, the fluorescence stability of HABP-mKate must be tested as follows:
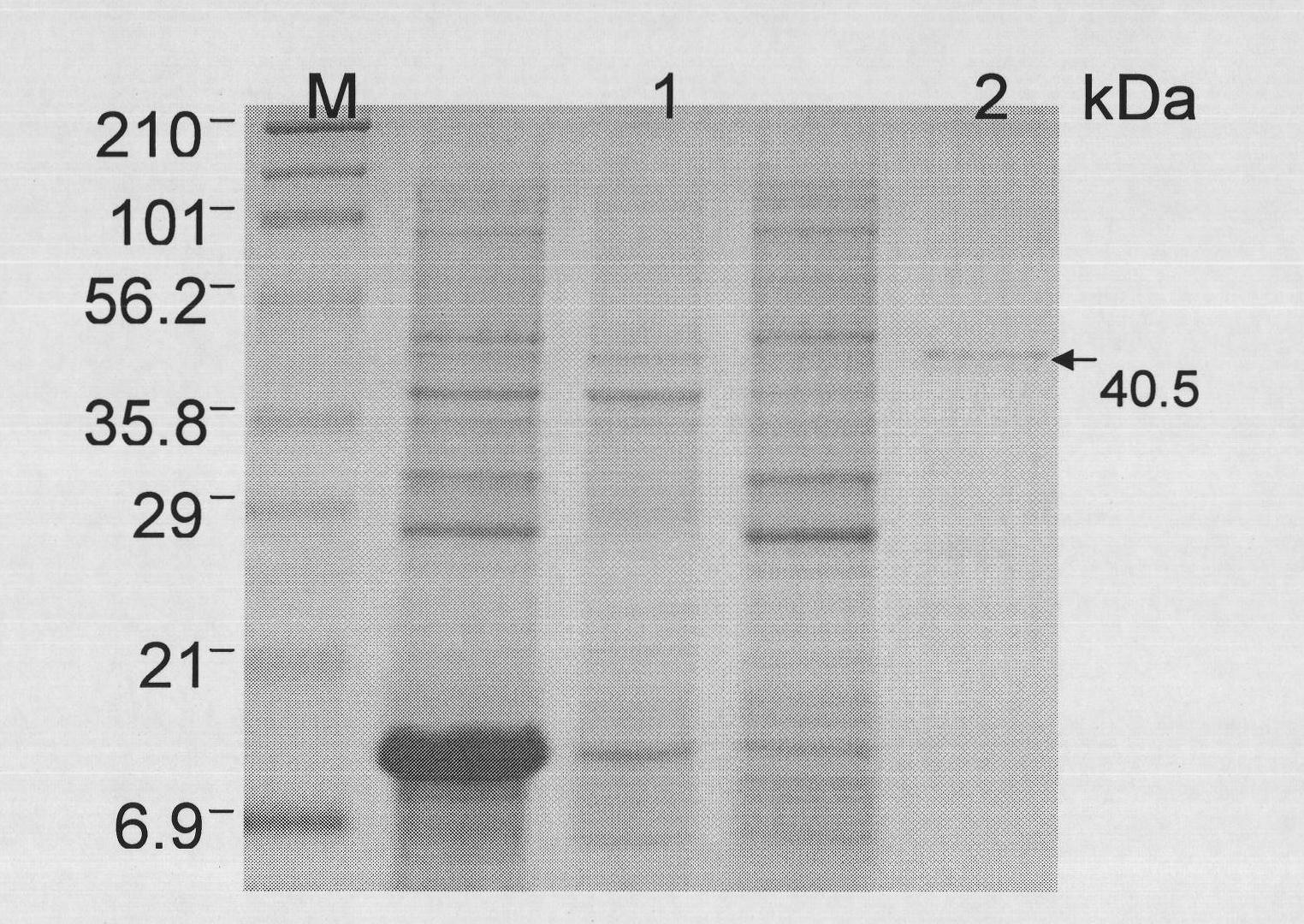
[0047] 1. Shake 1000ml of bacteria containing the HABP-mKate gene first, induce expression with 0.1M IPTG overnight at room temperature, suspend the collected bacteria in PBS, centrifuge after ultrasonic crushing to obtain the supernatant, and then filter the supernatant through a 0.45um filter. The resulting supernatant was purified by a nickel ion column, washed with PBS / 0.005M imidazole, eluted with PBS / 0.5M imidazole, and the protein concentration (OD280nm) was measured after dialysis (PBS, 10Kda membrane) after the elution, which was 0.2mg / ml
[0048] 2. Dilute HABP-mKate with a concentration of 0.2 mg / ml to a final concentration of 10 μg / m with PBS, take 100 μl and put it on a 96-well plate, and read the fluorescence value with a 96-well fluorescence pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com