Solid effervescent mixture for oral absorption
A technology for oral absorption and effervescent preparations, which is applied in anti-inflammatory agents, anti-toxic agents, food preparation, etc., can solve the problems of low bioavailability, slow drug effect onset, etc., achieve good taste, simple dosage form, and avoid liver first over effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: oral absorption solid energy effervescent preparation, the weight percentage of each component is:
[0057] Citric acid 5%, sodium bicarbonate 35%, calcium carbonate 15%, D-ribose 0.2%, glucuronolactone 0.3%, ginseng extract 0.5%, PEG 35%, aspartame 0.3%, mint flavor 0.6% , 4% sorbitol, 1% micronized silica gel, and the rest are colorants.
[0058] Preparation:
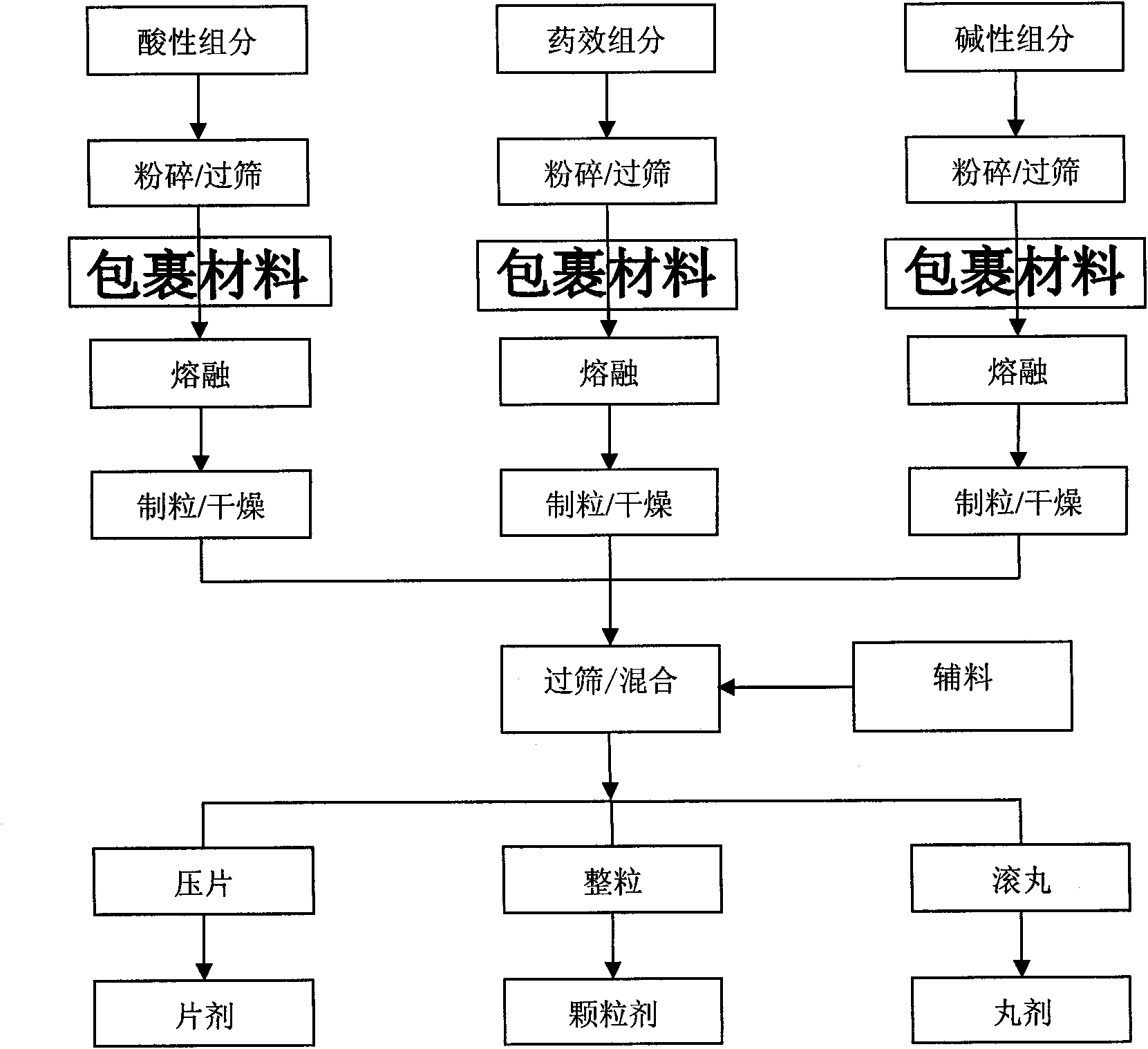
[0059] a. The above-mentioned citric acid, sodium bicarbonate, calcium carbonate, D-ribose, glucuronolactone, ginseng extract, and sorbitol were respectively pulverized through an 80-mesh sieve;
[0060] b. Mix citric acid, sorbitol and PEG in proportion, heat to 65°C to melt, granulate through a 20-mesh sieve, cool and dry, and set aside;
[0061] c. Mix sodium bicarbonate, calcium carbonate and PEG in proportion, heat to 65°C and melt, pass through a 20-mesh sieve to granulate, cool and dry, and set aside;
[0062] d. Mix D-ribose, glucuronolactone, ginseng extract, sorbitol and PEG in propor...
Embodiment 2
[0064] Embodiment 2: oral absorption solid energy effervescent preparation, the weight percentage of each component is:
[0065] Malic acid 50%, sodium carbonate 5%, natural caffeine 5%, ginseng extract 35%, PEG 0.5%, stevioside 0.3%, hawthorn powder 0.9%, PVP 3%, magnesium stearate 0.15%, the rest is coloring agent.
[0066] Preparation:
[0067] a. The above-mentioned malic acid, sodium carbonate, natural caffeine, and ginseng extract are pulverized respectively through an 80-mesh sieve;
[0068] b. Mix malic acid and PEG in proportion, heat to 65°C to melt, granulate through a 20-mesh sieve, cool and dry, and set aside;
[0069] c. Mix sodium carbonate and PEG in proportion, heat to 65°C to melt, granulate through a 20-mesh sieve, cool and dry, and set aside;
[0070] d. Mix natural caffeine, ginseng extract and PEG in proportion, heat to 65°C to melt, granulate through a 20-mesh sieve, cool and dry, and set aside;
[0071] e. Fully mix the components obtained in steps ...
Embodiment 3
[0072] Embodiment 3: oral absorption solid energy effervescent preparation, the weight percentage of each component is:
[0073] Tartaric acid 10%, calcium carbonate 30%, octacosanol 10%, D-ribose 10%, panthenol 16%, PEG 15%, spicy powder 0.8%, mannitol 7%, stevioside 0.2%, talc 0.95% , and the rest are colorants. Preparation:
[0074] a. above-mentioned tartaric acid, calcium carbonate, octacosanol, D-ribose, mannitol are pulverized respectively and cross 80 mesh sieves;
[0075] b. Mix tartaric acid with panthenol and PEG in proportion, heat to 90°C and melt, pass through a 20-mesh sieve to granulate, cool and dry, and set aside;
[0076] c. Mix calcium carbonate with panthenol and PEG in proportion, heat to 90°C and melt, pass through a 20-mesh sieve to granulate, cool and dry, and set aside;
[0077] d. Mix octacosanol, D-ribose, mannitol with panthenol and PEG in proportion, heat to 90°C and melt, pass through a 20-mesh sieve to granulate, cool and dry, and set aside; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com