Preparation method of slice-shaped B type carbonate phosphorite
A carbonate-type, apatite technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of poor biodegradability, harsh reaction conditions, long reaction time, etc. Effects of milder conditions, improved safety and energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Preparation of calcium carbonate powder: Calcium carbonate is mechanically ground to form a powder with a particle size of about 1 μm.
[0024] 2. Preparation of phosphate buffer solution: Dissolve sodium dihydrogen phosphate and disodium hydrogen phosphate in distilled water to prepare a phosphate buffer solution with pH=7.4 and total phosphate concentration of 0.2 mol / L.
[0025] 3. Preparation of flaky B-type carbonate-type apatite: Weigh 0.6 g of calcium carbonate powder, put it in 100 mL of phosphate buffer solution, heat it with non-pulse continuous microwave, power range 0~1200W, react at 80°C 5 min. The precipitate was collected by filtration, washed several times with deionized water, and dried in an oven at 80°C.
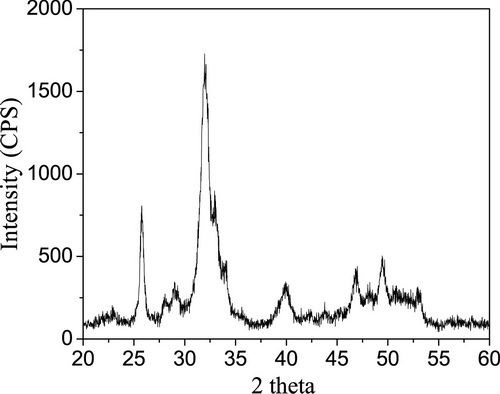
[0026] 4. Test: After scanning electron microscope inspection, the particle shape is flake. X-ray diffraction, Fourier transform infrared spectroscopy, and X-ray energy spectrometer detection showed that the sample was B-type carbonate-type apa...
Embodiment 2
[0028] 1. The fish bone is mechanically ground to form a fish bone powder with a particle size of about 2 μm.
[0029] 2. Dissolve sodium dihydrogen phosphate and disodium hydrogen phosphate in distilled water to prepare a phosphate buffer solution with a pH of 8.0 and a total concentration of phosphate of 0.3 mol / L.
[0030] 3. Preparation of flaky B-type carbonate-type apatite: Weigh 0.6 g of fish bone powder, of which the calcium carbonate content is 93%, put it in 100 mL of phosphate buffer solution, and heat it with pulsed microwave at a power of 100W , react at 100°C for 10 min. The precipitate was collected by filtration, washed several times with deionized water, and dried in an oven at 80°C.
[0031] 4. Test: The shape of the sample is sheet-like and the crystallinity is low by scanning electron microscopy; X-ray diffraction, Fourier transform infrared spectroscopy, and X-ray energy spectrometer detection show that the sample is B-type carbonate-type apatite, Ca / P ...
Embodiment 3
[0033] 1. First use a brush to remove impurities on the surface of the shell, and rinse with tap water. After sanding off the shell layer and prism layer, ultrasonication was performed for 5 min, and then cleaned with deionized water and absolute ethanol, and dried in a blast drying oven at 60 °C for 48 h to obtain a shiny shell nacre.
[0034] 2. Dissolve potassium dihydrogen phosphate and dipotassium hydrogen phosphate in distilled water to prepare a phosphate buffer solution with pH=10 and total phosphate concentration of 0.5 mol / L.
[0035] 3. Preparation of flaky B-type carbonate-type apatite: Weigh 2.0 g of shell nacre (in which calcium carbonate content is 98%), put it in 300 mL of phosphate buffer solution, and heat it with pulsed microwave at a power of 100W, 20 min at 100°C. The precipitate was collected by filtration, washed several times with deionized water, and dried in an oven at 80°C.
[0036] 4. Test: X-ray diffraction, Fourier transform infrared spectroscop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com