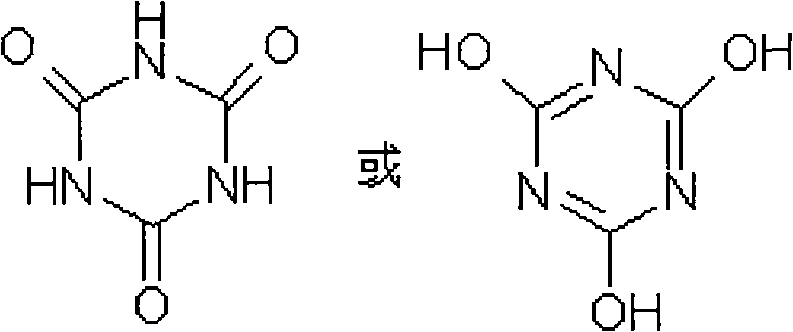
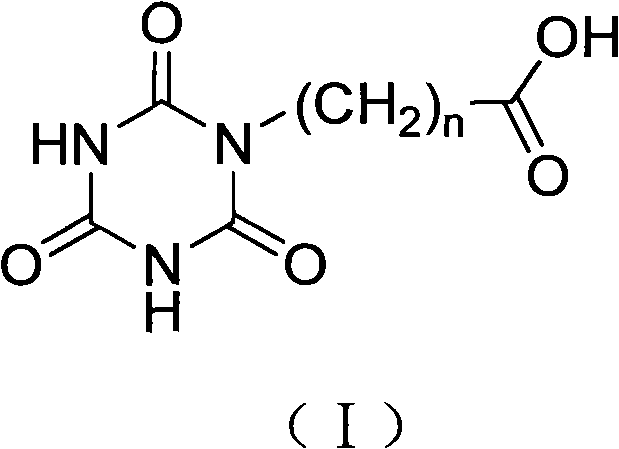
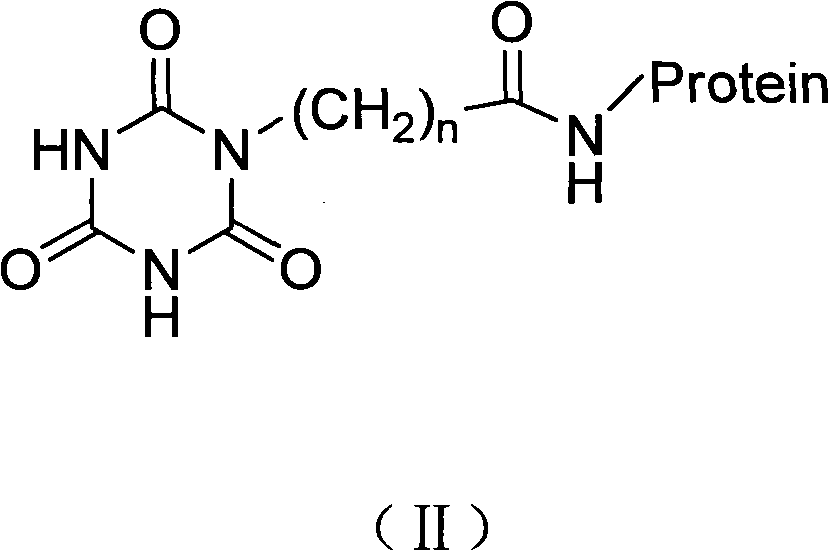
Cyanurate hapten, artificial antigen and antibody, and preparation method and applications thereof
A technology of artificial antigen and cyanuric acid, which is applied in the field of food safety, can solve the problems of complex and difficult preparation technology of hapten substance antibody, and achieve the effect of avoiding instrument analysis steps, simple operation and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Hapten Synthesis
[0050] This embodiment takes benzyl bromoacetate when n=1 as an example.
[0051] Put 5mmol cyanuric acid and 5mmol benzyl bromide into a round bottom flask, add 8mL DMF, then add 5mmol DMAP, react at 40-80°C for 10h, evaporate the solvent under reduced pressure, dissolve the residue with tetrahydrofuran, filter, and concentrate the filtrate to Dry, use dichloromethane+methanol (95+5) as the developing solvent, column chromatography, collect the component with Rf=0.3, evaporate the solvent, the white solid is hydrolyzed with excess potassium hydroxide overnight, and then acidified to pH with concentrated hydrochloric acid The value was 3, the solvent was evaporated to dryness, and a white solid appeared, which was washed with cold water for 3 to 5 times to obtain the target hapten.
[0052] When n=3~8, the method is basically the same as above, only the length of the carbon chain in the benzyl bromide is replaced, that is, the corresponding...
Embodiment 2
[0053] Embodiment 2 artificial antigen preparation
[0054] In this embodiment, the artificial antigen prepared from the hapten obtained by the benzyl bromoacetate method when n=1 is taken as an example is described as follows.
[0055] Active ester method: dissolve 0.20 mmol of hapten in 1.0 mL of dioxane (or dimethylformamide), add 0.22 mmol of dicyclohexylcarbodiimide and 0.22 mmol of N-hydroxysuccinimide, React at room temperature for 3.5 hours, centrifuge to remove the precipitate, drop the supernatant into 5 mL of carbonic acid buffer solution with a pH of 7.4-9.55, the buffer solution contains 10 mg / mL carrier protein, and the carrier protein is BSA; after reaction for 2-4 hours, put the reaction solution into Put it into a dialysis bag, and dialyze it with 0.85% normal saline for 3 days to obtain an artificial antigen (named: M-a-3-BSA).
[0056] The preparation process of replacing the carrier protein with KLH or CONA or THY or OVA can be understood by those skilled ...
Embodiment 3
[0057] Example 3 Artificial Antigen Preparation
[0058] Mixed anhydride method: Dissolve 0.1 mmol of hapten in 1.5 mL of dioxane, add 0.15 mmol of isobutyl chloroformate, stir at 4°C for 0.5 h, and this is liquid A. Dissolve 50 mg of carrier protein in 4 mL of 1 mol / L sodium bicarbonate buffer at pH 9.6, which is solution B. The carrier protein is OVA. Slowly add solution A to solution B dropwise, stir and react overnight at 4°C, put the reaction solution into a dialysis bag, and dialyze with 0.85% normal saline for 3 days to obtain artificial antigen.
[0059] When n=2~8, the artificial antigen preparation method is the same as n=1, only the length of the carbon chain in the benzyl bromide is replaced during implementation, that is, the hapten obtained by the corresponding benzyl bromide reaction is adopted, and the molar ratio The hapten obtained by the benzyl bromoacetate method will not be repeated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com