Method for preparing intermediate of cilastatin
A technology for cilastatin and intermediates, which is applied to the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve the problem of low yield of cilastatin, and achieve simple preparation engineering, high yield, The effect of shortening the time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
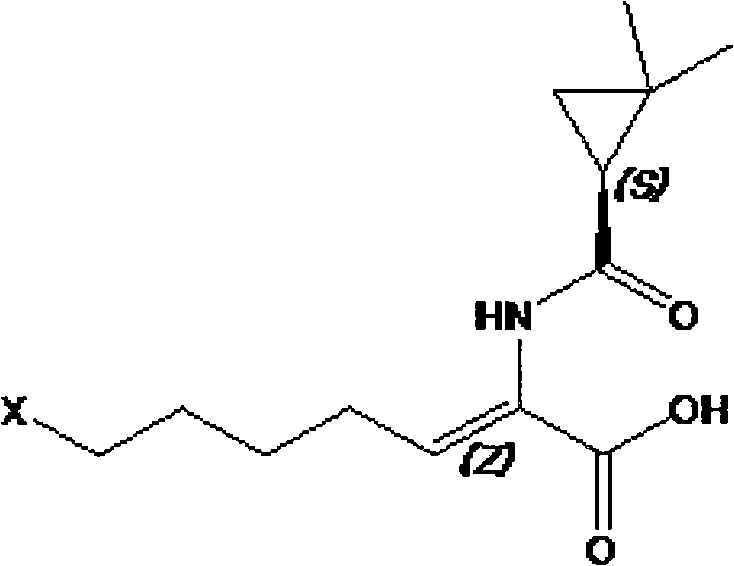
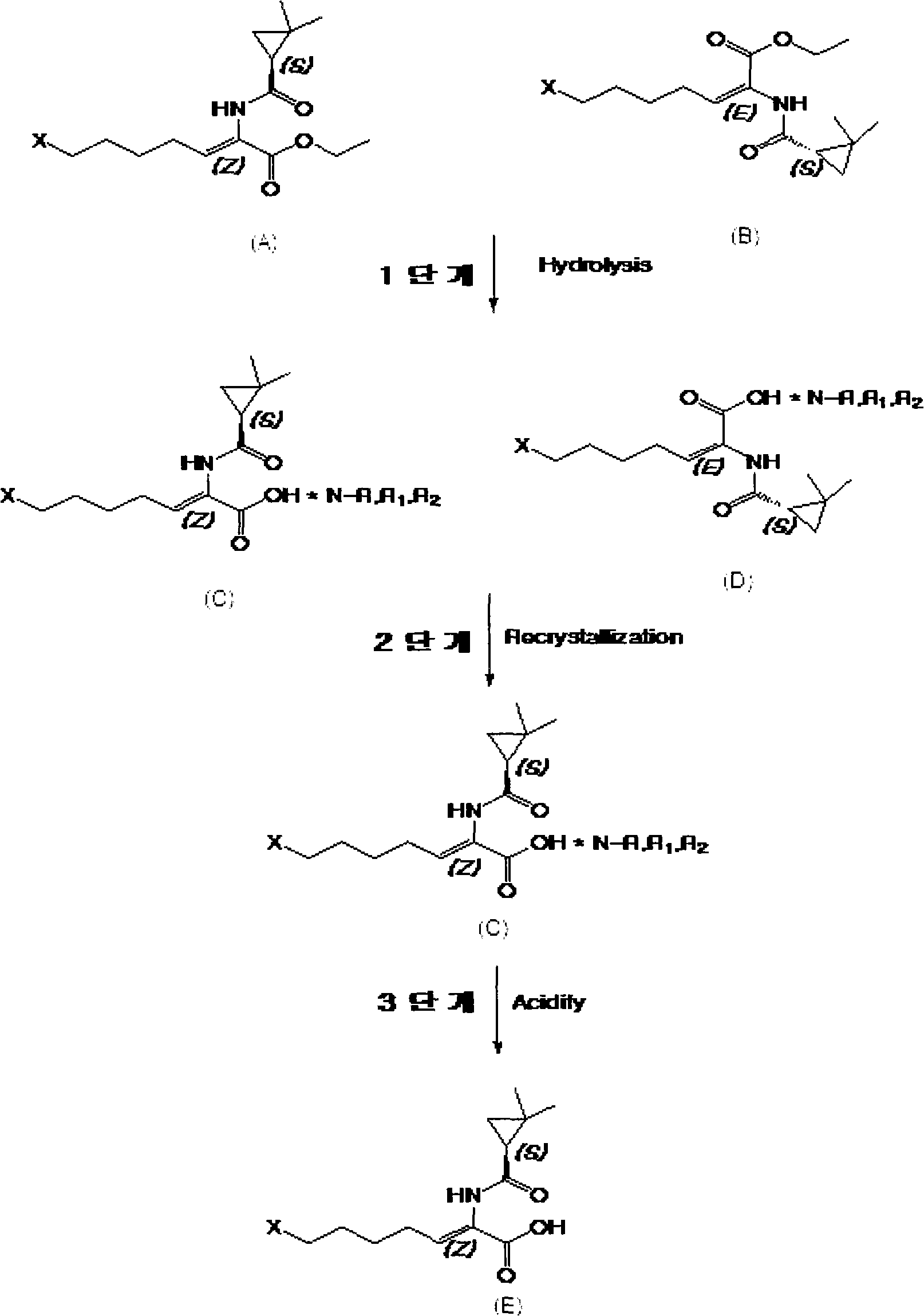
[0019] The preparation method of cilastatin of the present invention can be generalized with [reaction formula 1].
[0020] Reaction 1
[0021]
[0022] Here, X refers to a halogen element such as fluorine or chlorine.
[0023] With reference to the first stage of the above [reaction formula 1], in (A) considered as [reaction formula 1], (E, Z)-7-halogen-2-((S)-2,2-di Add alkaline solution to methylcyclopropaneamido)-2-heptenoic acid ethyl ester.
[0024] (E, Z)-7-halo-2-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ethyl ester can be prepared by the method described in European Patent Publication No. 48301 , In addition, although there is no record, it can also be prepared by many methods.
[0025] The base used here may be sodium hydroxide, sodium carbonate, potassium hydroxide, potassium carbonate or lithium hydroxide.
[0026] Add base to (A) and (B) of [Reaction Formula 1] for hydrolysis to obtain (E, Z)-7-halogen-((S)-2,2-dimethylcyclopropaneamido)-2 - an ...
reference example 1
[0044] Preparation of (E, Z)-7-chloro-2-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ethyl ester
[0045] With reference to the method described in European Patent Publication No. 48301, 25.0 g (0.1209 mol) of 7-chloro-2-oxo-heptenoic acid ethyl ester, (S)-2,2-dimethylcyclopropaneamide 13.68 g (0.1209mol), 125ml of toluene and 0.25g of methyl sulfuric acid were put into a 500ml flask, and refluxed for 23 hours. The toluene was then evaporated to give ethyl (E,Z)-7-halo-2-((S)-2,2-dimethylcyclopropanamido)-2-heptenoate.
experiment example 1
[0047] Stage 1: Hydrolysis of (E, Z)-ethyl 7-chloro-2-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoate and preparation of (E, Z) -7-Chloro-2-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid n-propylamine salt.
[0048] At room temperature, add (E, Z)-7-chloro-2-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid ethyl ester obtained in Reference Example 1 50ml of water and 15.1g of sodium hydroxide, at the same temperature, through 24 hours of stirring, to obtain the isomer (Z)-7-chloro-2-( (S)-2,2-Dimethylcyclopropaneamido)-2-heptenoic acid sodium salt aqueous solution.
[0049] Add 150ml of toluene to (E, Z)-7-chloro-2-((S)-2,2-dimethylcyclopropaneamido)-2-heptenoic acid sodium salt solution, and use chloric acid aqueous solution to The pH was adjusted to 3. After separation, anhydrous magnesium sulfate was added to the organic solvent layer and then filtered. In the remaining liquid, add 8.93g of n-propylamine, after stirring for 1 hour, rotary distillation of tolu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com