Nux vomica capsules for treating myasthenia gravis and preparation process thereof
A technology of myasthenia gravis and preparation process, which is applied in the direction of capsule delivery, medical preparations containing active ingredients, muscular system diseases, etc., and can solve the problem of narrow application range, no treatment method and short action time of plasma exchange and thymectomy To achieve the effect of ensuring the safety of clinical medication, good patient compliance, and strong application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Processing of Nuxychnium Capsules
[0031] Nymph 20kg Sesame Oil 18kg Liuyisan 15kg
[0032] (1) Nuxychnya raw medicinal material, remove impurities, sieve to remove ash, soak in water for 10 days until there is no hard core inside, remove, scrape off fur, dry slightly, cut into thin slices, and dry in the air. (2) Heat sesame oil in a pan, add dried flakes, fry until yellow-brown, take out, sprinkle Liuyisan evenly, absorb sesame oil, rinse Liuyisan with clean water, dry in the sun, and set aside. (3) Beat into fine powder with a 100-mesh sieve, sterilize, and fill into capsules.
Embodiment 2
[0034] Quality Control of Nuxebra Capsules
[0035] (1) This product is a hard capsule, and each capsule contains 0.2g of roasted nuyon seed powder, and the difference in the filling amount should be within 10%.
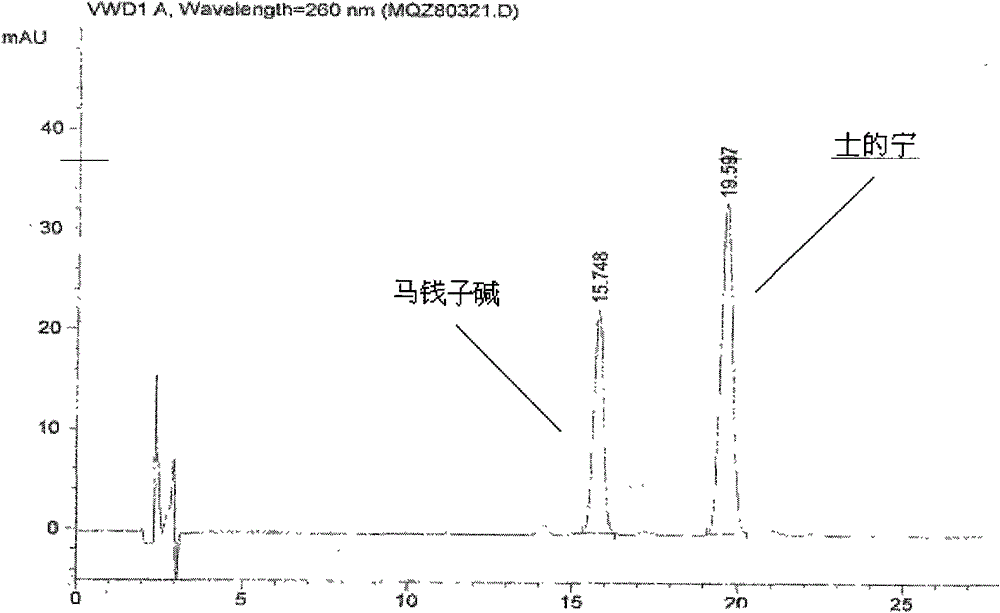
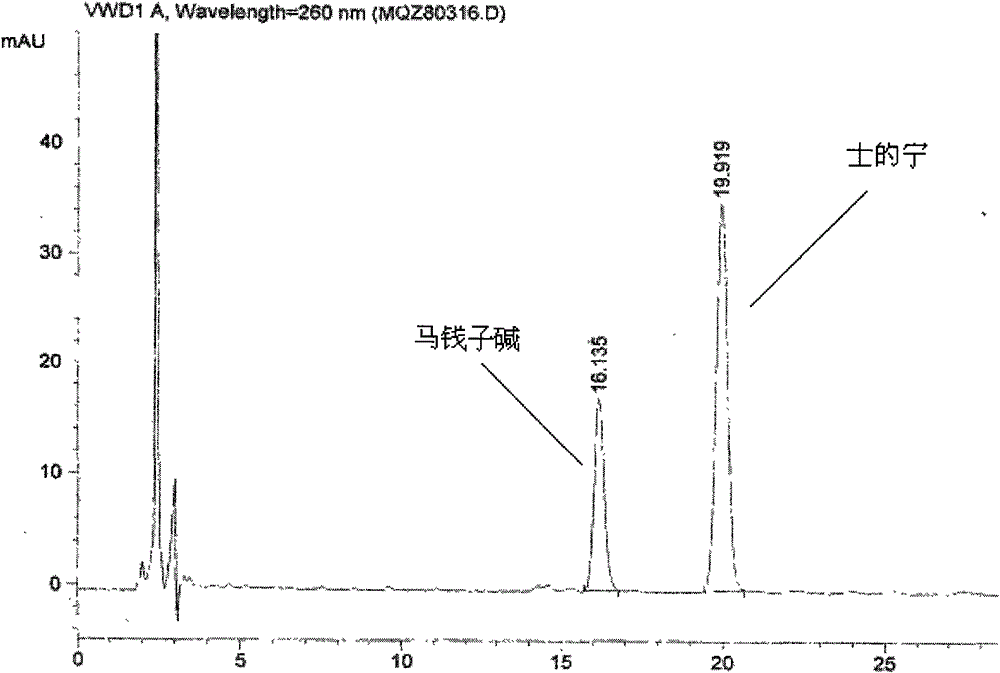
[0036] (2) Identification: Take 0.5g of the contents of this product, add 0.5ml of concentrated ammonia test solution and mix well, add 5ml of chloroform-ethanol (10:1) mixed solution, seal it tightly, shake for 5 minutes, and let it stand for 2 hours , filtered, and the filtrate was used as the test solution. Take another strychnine reference substance and strychnine reference substance, add chloroform to make a mixed solution containing 2mg per 1ml, as the reference substance solution. Test according to thin-layer chromatography (Appendix VIIB of "Chinese Pharmacopoeia" 2005 edition), draw 10 μl of each of the above two solutions, spot them on the same silica gel G plate respectively, and use toluene-acetone-ethanol-concentrated ammonia test solution (4: 5∶0.6∶0....
Embodiment 3
[0049] Clinical Observation on Myasthenia Gravis Treated by Nuxynum Capsules
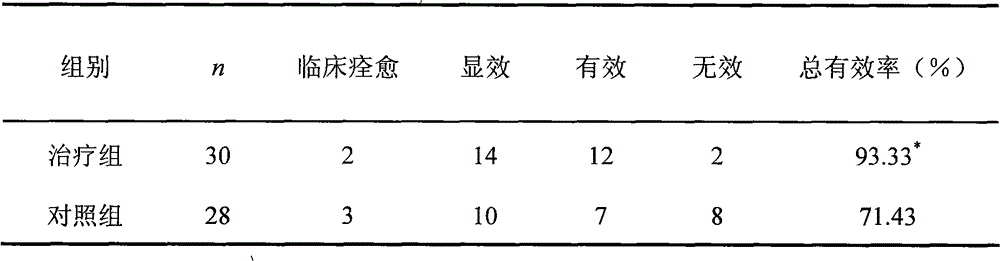
[0050] (1) Case selection and group observation There were 58 cases, 36 males and 22 females; the youngest was 20 years old, the oldest was 65 years old; the shortest course of disease was 24 days, and the longest was 21 years; 34 cases of ocular muscle type, There were 2 cases of medullary type, 4 cases of spinal type, and 18 cases of generalized type. They were divided into 31 cases in Nuxebra Capsules group and 27 cases in Pyridostigmine bromide control group. There was no significant difference between the two groups (age, gender, disease duration) (p>0.05).
[0051] (2) Therapeutic medication treatment group: treated with Nuxebra Capsules, 2 capsules each time, orally 3 times a day, if the patient was taking pyridostigmine bromide, prednisone or acetone, continue to take them, wait until immediately After taking Qianzi Capsules for 10-20 days, gradually reduce the dosage until completely disc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com