Cathode material of solid oxide fuel cell and preparation method thereof
A fuel cell cathode, solid oxide technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of inconsistent thermal matching of LSGM electrolyte and high thermal expansion coefficient of LSFO system, and achieve short preparation cycle, small particle diameter, speed up The effect of speed of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
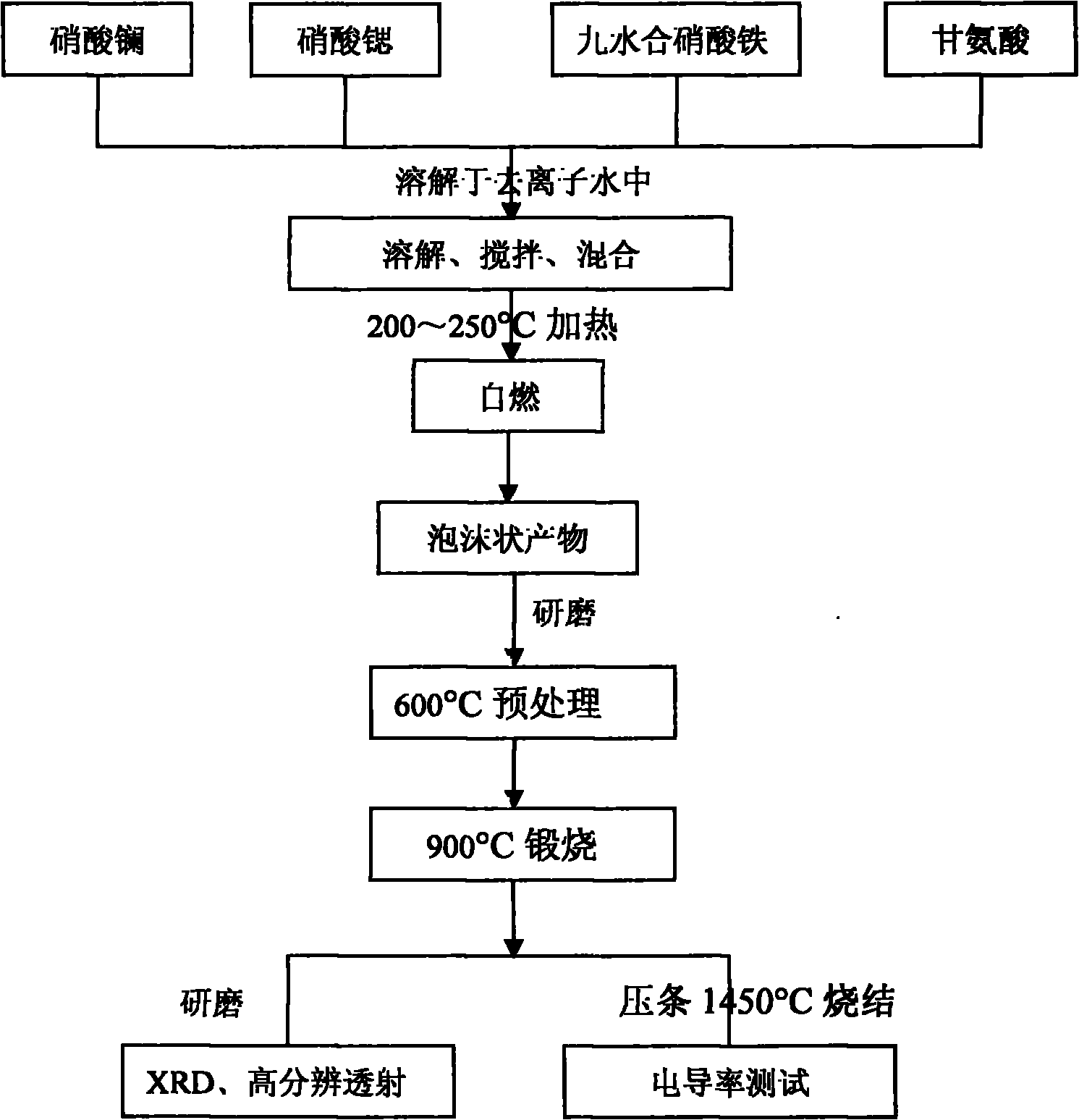
[0021] Example 1: 0.04mol La 1.2 Sr 0.8 FeO 4±δ Synthesis of Glycine Method and Its Conductivity Test
[0022] 1. Weigh 0.048mol of lanthanum nitrate into a beaker, add deionized water, heat on an electric furnace at 200°C, stir, and dissolve to form a colorless transparent solution.
[0023] 2. Weigh 0.032mol of strontium nitrate, put it into a beaker, add deionized water, and ultrasonically disperse it to completely dissolve it.
[0024] 3. Weigh 0.04mol of ferric nitrate nonahydrate, add deionized water and ultrasonically disperse to completely dissolve
[0025] 4. Weigh 0.24 mol of glycine, add deionized water and ultrasonically disperse to dissolve completely.
[0026] 5. Mix the above-mentioned transparent solution evenly, heat to 230°C in an evaporating dish, and evaporate the excess water until it spontaneously ignites to form a porous foamy powder.
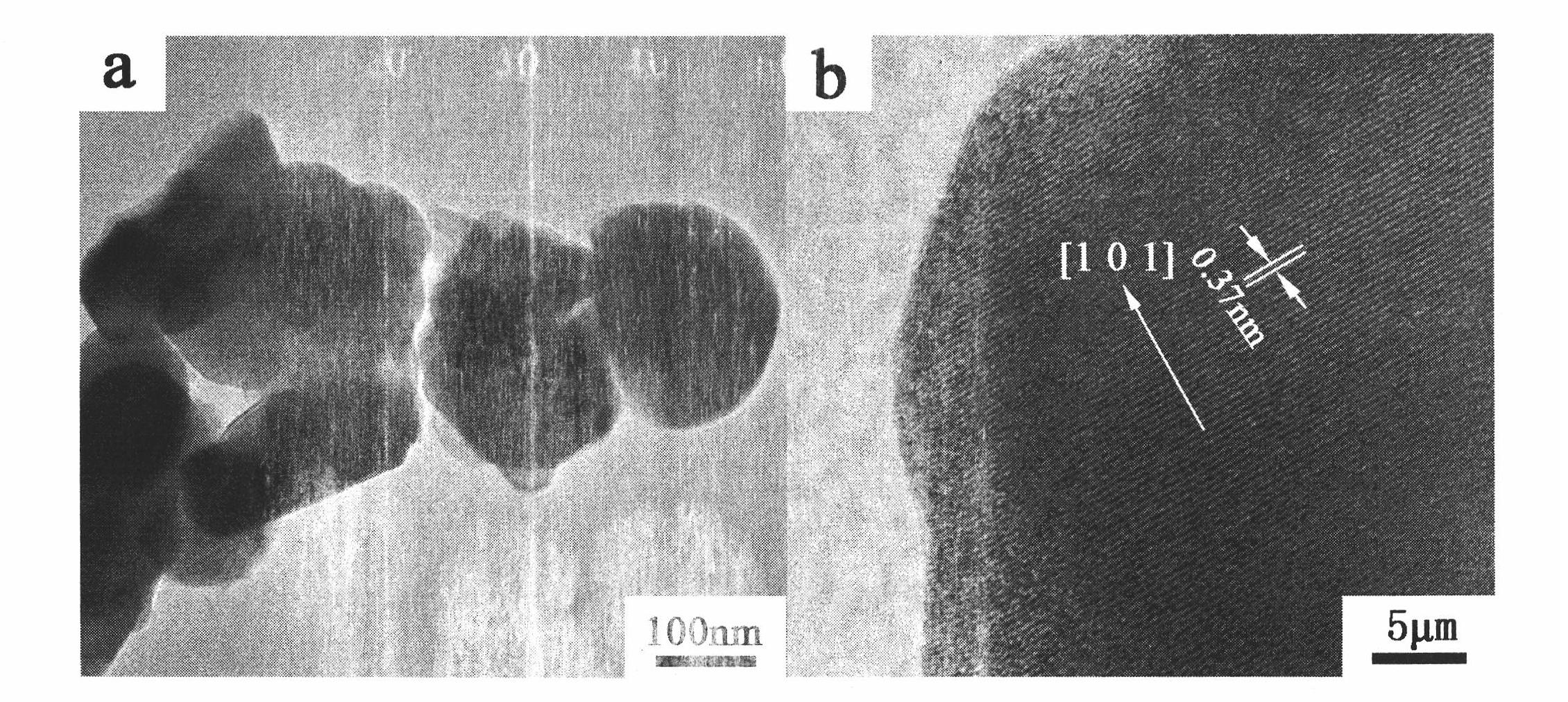
[0027] 6. After the foamy powder is pretreated at 600°C, it is ground, and a part of the powder is taken out and c...
Embodiment 2
[0030] Example 2: 0.08mol La 1.2 Sr 0.8 FeO 4±δ Synthesis of Glycine Method and Its Conductivity Test
[0031] 1. According to La 1.2 Sr 0.8 FeO 4±δ For the stoichiometric ratio of substances, weigh 0.096mol of lanthanum nitrate, 0.064mol of strontium nitrate, and 0.08mol of ferric nitrate nonahydrate, and dissolve them in deionized water to form a colorless transparent solution.
[0032] 2. According to the ratio of glycine to metal ion is 1.8, weigh 0.432 mol of glycine, add deionized water and ultrasonically disperse to dissolve completely.
[0033] 3. Mix the above transparent solution evenly, stir and heat to 200°C in an evaporating dish, evaporate the excess water until it spontaneously ignites to form a porous foamy powder.
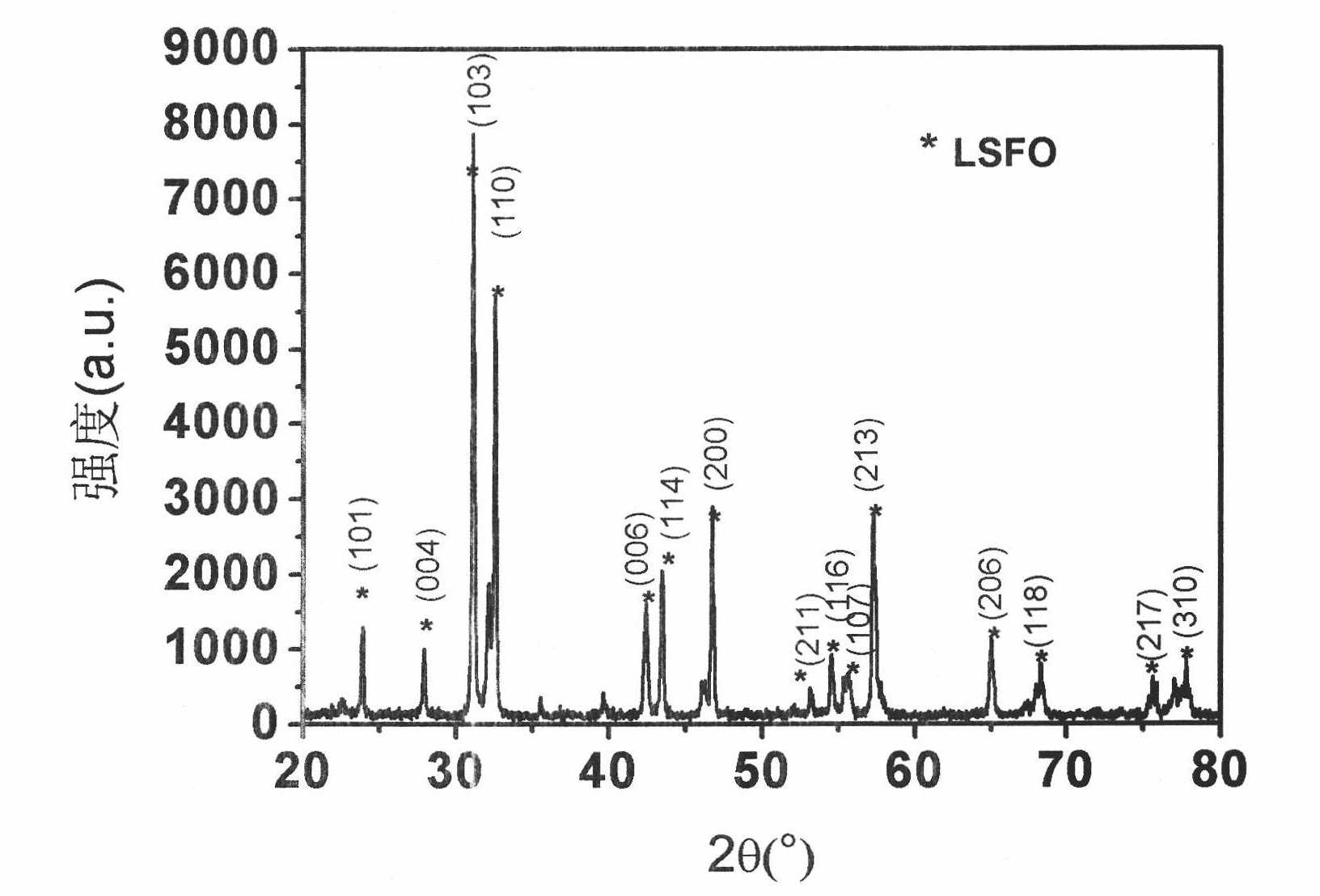
[0034] 4. After the foamy powder is pretreated at 500°C, it is ground, and a part of the powder is taken out and calcined at 950°C for 10b, cooled to room temperature, ground, and tested by X-ray diffraction.
[0035] 5. Grind the pretreated...
Embodiment 3
[0037] Example 3: 0.02mol La 1.2 Sr 0.8 FeO 4±δ Synthesis of Glycine Method and Its Conductivity Test
[0038] 1. According to La 1.2 Sr 0.8 FeO 4±δ The stoichiometric ratio of the metal ions, weighing 0.024mol of lanthanum nitrate, 0.016mol of strontium nitrate, 0.02mol of iron nitrate nonahydrate, according to the ratio of glycine and metal ions is 2.2, weighing 0.132mol of glycine, dissolved into Ionized water, ultrasonically dispersed to make it completely dissolved, forming a colorless and transparent solution, heated to 250 ° C in an evaporating dish, evaporated to dry excess water, until spontaneous combustion, forming a porous foamy powder.
[0039] 2. Pretreat the foamy powder at 650°C, grind it, take out a part of the powder and calcinate at 1000°C for 8 hours, cool to room temperature, grind it, and do X-ray diffraction test.
[0040] 3. Grind the powder after pretreatment at 650°C, add it to the mold, hold the pressure for 35 minutes under the gauge pressure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com