Preparation method of aryl propionic acid derivative
A technology of arylpropionic acid and derivatives, applied in the field of preparation of arylpropionic acid derivatives, can solve the problems of harsh reaction conditions, lengthy routes, and high requirements for production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
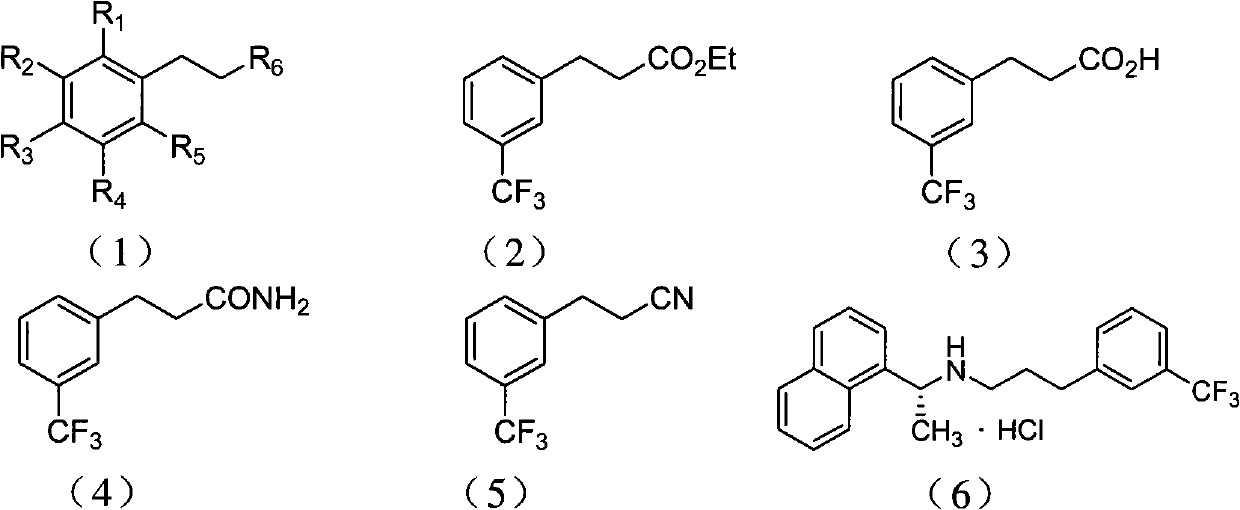
[0054] Embodiment 1: the preparation of 3-(3-(trifluoromethyl)phenyl)propionic acid
[0055] Preparation of Diethyl 2-(3-(trifluoromethyl)benzyl)malonate
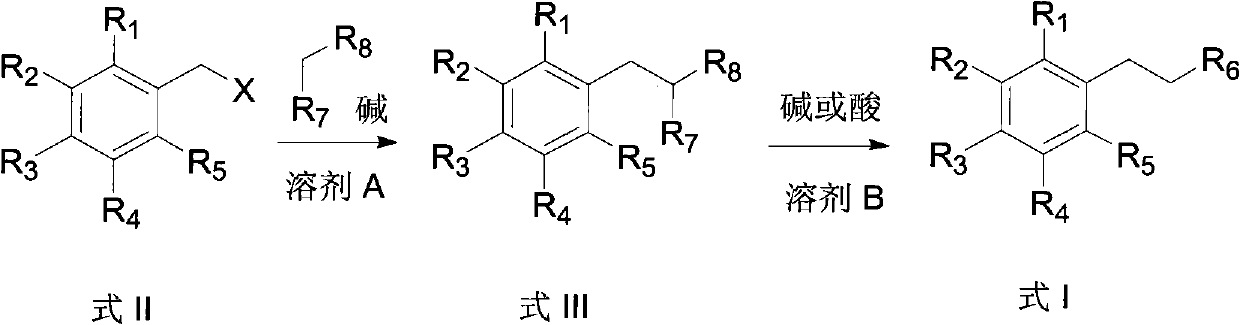
[0056] Under nitrogen protection, m-trifluoromethylbenzyl chloride (19.5 g, 0.1 mol), diethyl malonate (16 g, 0.1 mol), 100 ml of acetonitrile and potassium carbonate (27.6 g, 0.2 mol) were added to the reaction In the bottle, the reaction was stirred at reflux for 48 hours. After the reaction, cool to room temperature, evaporate acetonitrile, add 100 ml of water and 100 ml of ethyl acetate, extract and separate the organic layer, and extract the aqueous layer twice with 100 ml of ethyl acetate. All organic layers were combined and washed with 50 mL of saturated aqueous sodium chloride. Then the organic layer was dried over anhydrous sodium sulfate, concentrated, and the resulting oil was separated by silica gel column chromatography (developing solvent: petroleum ether / ethyl acetate 20:1, volume ratio) to obtain 2-(3-(tr...
Embodiment 2
[0059] Embodiment 2: the preparation of 3-(3-(trifluoromethyl)phenyl) propionic acid
[0060] Preparation of 2-(3-(trifluoromethyl)benzyl)malononitrile
[0061] Under nitrogen protection, m-trifluoromethylbenzyl chloride (19.5 g, 0.1 mol), malononitrile (6.6 g, 0.1 mol), 100 ml of toluene and potassium carbonate (27.6 g, 0.2 mol) were added to the reaction flask, The reaction was stirred at reflux for 1 hour. After the reaction, cool to room temperature, evaporate toluene, add 100 ml of water and 100 ml of ethyl acetate, separate the organic layer, and extract the water layer twice with 100 ml of ethyl acetate. All organic layers were combined and washed with 50 mL of saturated aqueous sodium chloride. The organic layer was dried over anhydrous sodium sulfate, concentrated, and the resulting oil was separated by silica gel column chromatography (developing solvent: petroleum ether / ethyl acetate 20:1, volume ratio) to obtain 2-(3-(trifluoromethyl ) benzyl) malononitrile 22.2...
Embodiment 3
[0064] Embodiment 3: the preparation of 3-(3-(trifluoromethyl)phenyl)propionitrile
[0065] Preparation of ethyl 2-cyano-3-(3-(trifluoromethyl)phenyl)propionate
[0066]Under nitrogen protection, m-trifluoromethylbenzyl chloride (19.5 g, 0.1 mol), ethyl cyanoacetate (11.3 g, 0.1 mol), 100 ml of toluene and sodium carbonate (21.2 g, 0.2 mol) were added to the reaction flask In, the reaction was stirred at reflux for 12 hours. After the reaction, cool to room temperature, evaporate toluene, add 100 ml of water and 100 ml of ethyl acetate, separate the organic layer, and extract the water layer twice with 100 ml of ethyl acetate. All organic layers were combined and washed with 50 mL of saturated aqueous sodium chloride. The organic layer was dried over anhydrous sodium sulfate, concentrated, and the resulting oil was separated by silica gel column chromatography (developing solvent: petroleum ether / ethyl acetate 20:1, volume ratio) to obtain 2-cyano-3-(3- (Trifluoromethyl)phe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com