Application of Pd catalyst in hydrogenation process for producing doxycycline
A doxycycline and production process technology, applied in the application field of chiral asymmetric hydrogenation production process, can solve the problems of inability to separate and recycle catalysts, low catalyst preparation yield, unfavorable industrial production, etc., and achieve high catalytic activity and three-dimensional Selective, beneficial to environmental protection, beneficial to the effect of industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: preparation palladium supported catalyst
[0022] Synthesis of polyvinyl chloride polyvinyl polyamine-loaded palladium complex: In a three-necked flask equipped with an electric stirrer, a reflux condenser and a thermometer, add 5.0 grams of pvc and 20 mL polyvinyl polyamine, swell overnight, and then place it in a boiling water bath Heating and stirring in medium temperature for 2 hours, cooling, stirring with water (heat generation), cooling, suction filtration, washing with water until neutral and colorless, then washing with ethanol until the ethanol is colorless, vacuum drying to constant weight, to obtain brown polyvinyl chloride polyvinyl chloride Polyamines. IR: 3339.98, 1584.72, 1428.72, 1252.52, 1120.91 cm-1.
[0023] Preparation of palladium-supported catalyst: Weigh 2.0 g of the above-mentioned polyvinyl chloride polyethylene polyamine, dissolve 0.1 g of palladium dichloride in 50 ml of acetone (ultrasonic dissolution), put them together in a ...
Embodiment 2
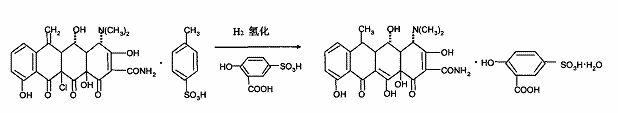
[0024] Embodiment 2: Doxycycline is prepared by 11α-chloro-6-methylene oxytetracycline p-toluenesulfonate
[0025] Take 100 grams of 11α-chloro-6-methylene oxytetracycline p-toluenesulfonate, and dissolve 0.15 grams of the above-prepared catalyst pvc-pp-pd in 300 mL of ethanol, and place them in a reactor with hydrogen gas and stir for reaction. The reaction temperature is 50°C and the pressure is 0.4-0.5Mpa.
[0026] Analysis by high-pressure liquid chromatography showed the progress of the reaction. After the reaction, filter and recover the catalyst, add 50.85 g of solid sulfosalicylic acid to the filtrate to form a salt, stir the reaction and cool, filter, and use 1:1 (volume ratio) ethanol: Washed with aqueous solution and dried. 92.1 g (0.139 mol) of a-6-deoxyoxytetracycline sulfosalicylate was obtained, with a yield of 90.2%. The isomer content of β-6-deoxyoxytetracycline sulfosalicylate is 0.6%.
Embodiment 3
[0027] Embodiment 3: 11α-chloro-6-methylene oxytetracycline p-toluenesulfonate prepares doxycycline
[0028] Take 100 g of 11α-chloro-6-methylene oxytetracycline p-toluenesulfonate, add 0.08 g of the above-prepared catalyst pvc-pp-pd, dissolve in 250 ml of methanol, place in a reactor and pass through hydrogen to stir the reaction. The reaction temperature is 35°C, and the reaction pressure is 0.6-0.65Mpa.
[0029] Analysis by high pressure liquid chromatography showed the progress of the reaction. After the reaction, filter and recover the catalyst, add solid sulfosalicylic acid to the filtrate to form a salt of 63.56g, stir the reaction and cool, filter, wash with 50ml of 50% ethanol, and dry. 95.1 g of α-6-deoxyoxytetracycline sulfosalicylate was obtained, the yield was 92.8%, and the isomer content of β-6-deoxyoxytetracycline sulfosalicylate was 0.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com