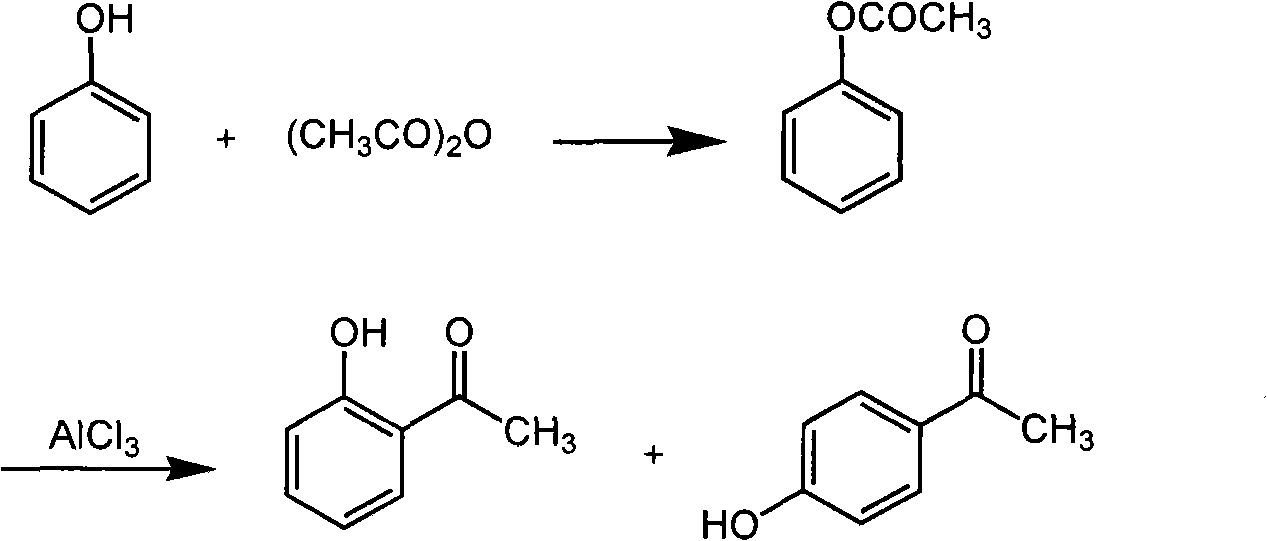
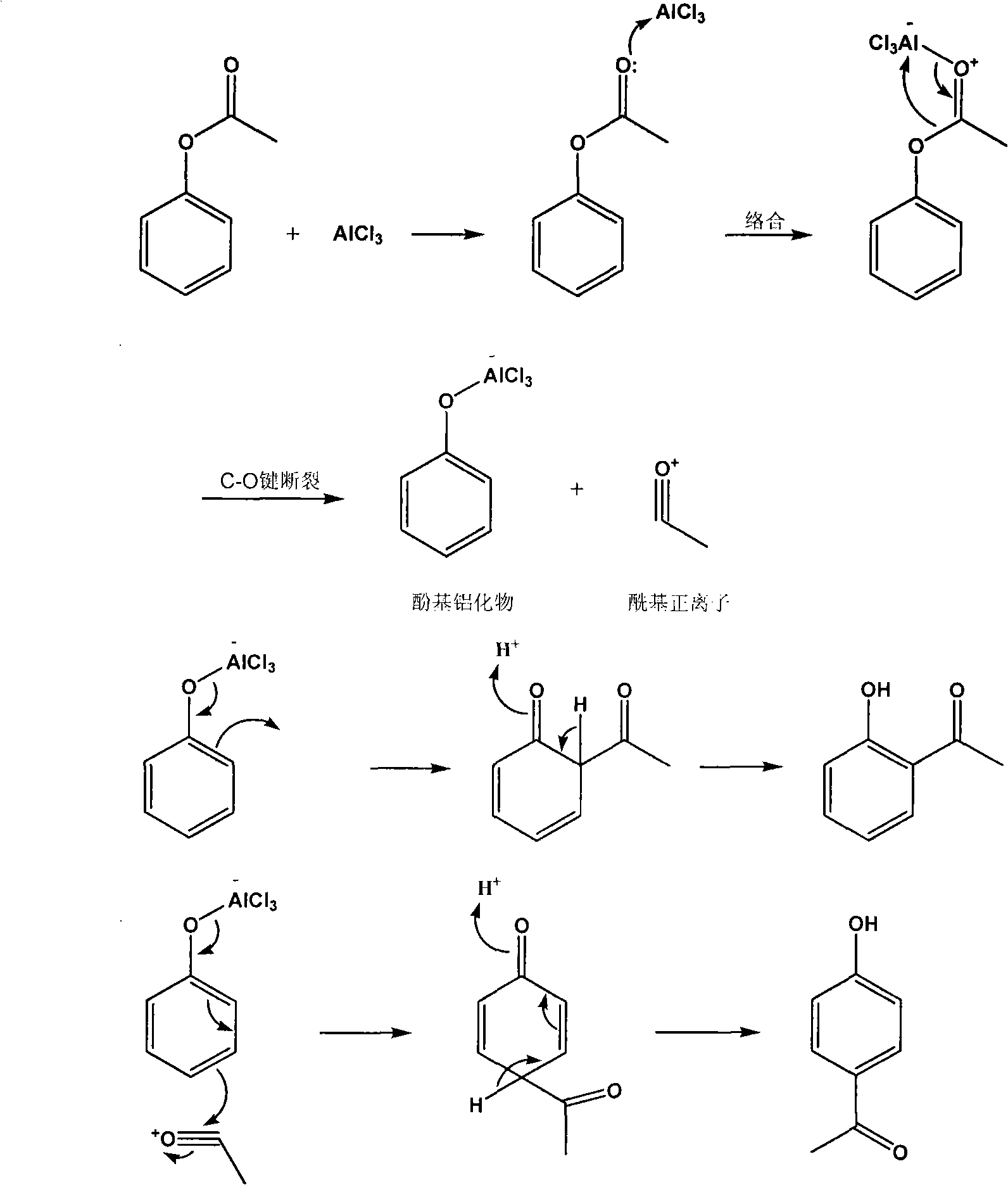
Method for preparing o-hydroxyacetophenone and p-hydroxyacetophenone
A technology for p-hydroxyacetophenone and o-hydroxyacetophenone, which is applied in the field of preparation of o-hydroxyacetophenone and p-hydroxyacetophenone, can solve the problems of complicated post-treatment, serious environmental pollution and the like, and achieves mild experimental conditions, The effect of high experimental yield and good promotion and application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Preparation of ethyl phenol phenol ester
[0023] Mix 9.1g of phenol and 107g of acetic anhydride in a flask evenly, place in a cold bath, and add 1 drop of concentrated sulfuric acid dropwise. Shake and cool down to room temperature, distill acetic acid under normal pressure, and then collect fractions at 194-196°C to obtain ethyl phenol phenol ester with a yield of 98% (calculated as phenol).
[0024] (2) preparation of p-hydroxyacetophenone and o-hydroxyacetophenone
[0025] Mix 74.8g of aluminum trichloride and 30.5g of phenol acetate, slowly heat to 160°C in an oil bath, and keep it warm for 2 hours. When cooled to 40-50°C, add glacial hydrochloric acid solution (220mL, 3.2mol / L) for hydrolysis, separate the organic phase, freeze, and filter to obtain crude p-hydroxyacetophenone.
[0026] The filtrate was steam distilled, and the organic phase separated from the distillate was distilled under reduced pressure to obtain a light yellow oily liquid o-hydroxyacet...
Embodiment 2
[0028] (1) Preparation of ethyl phenol phenol ester
[0029] Mix 94g of phenol with 110g of acetic anhydride, heat to reflux for 30 minutes, remove acetic acid by fractional distillation, and collect fractions at 194-196°C to obtain phenol acetate with a yield of 95% (calculated as phenol).
[0030] (2) preparation of p-hydroxyacetophenone and o-hydroxyacetophenone
[0031] Put 74.8g of aluminum trichloride in 80mL of carbon disulfide, control the speed of dropping phenol acetate (68g) under stirring, and keep the system in a slightly boiling state. After the dropwise addition, the mixture was heated under reflux in a water bath for 2 hours, then the solvent was evaporated, heated in an oil bath, and stirred at 175°C for 3 hours. After cooling to room temperature, hydrochloric acid solution (220 mL, 3.2 mol / L) was added for hydrolysis, and the organic phase was separated and subjected to steam distillation to obtain o-hydroxyacetophenone (24.5 g, 36%). The residue was extrac...
Embodiment 3
[0033] (1) Preparation of ethyl phenol phenol ester
[0034] Mix 94g of phenol with 110g of acetic anhydride, heat to reflux for 30 minutes, remove acetic acid by fractional distillation, and collect fractions at 194-196°C to obtain phenol acetate with a yield of 95% (calculated as phenol).
[0035] (2) preparation of p-hydroxyacetophenone and o-hydroxyacetophenone
[0036] Mix 60g of sodium chloride and 136g of powdered aluminum trichloride in a bottle evenly, heat to 230°C and keep for 1 hour. Add 100 g of phenol acetate dropwise within 30 minutes at about 200°C, and react at 240°C for 10 minutes after the dropwise addition is complete. Cool to room temperature, add hydrochloric acid solution (300mL, 10%) for hydrolysis, steam distillation, extract the distillate with ether, dry the extract to recover ether, then distill under reduced pressure, collect fractions at 101-105°C / 2KPa to obtain o-hydroxybenzene Ethyl ketone (42 g, 42%). The steam distillation residue was extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com