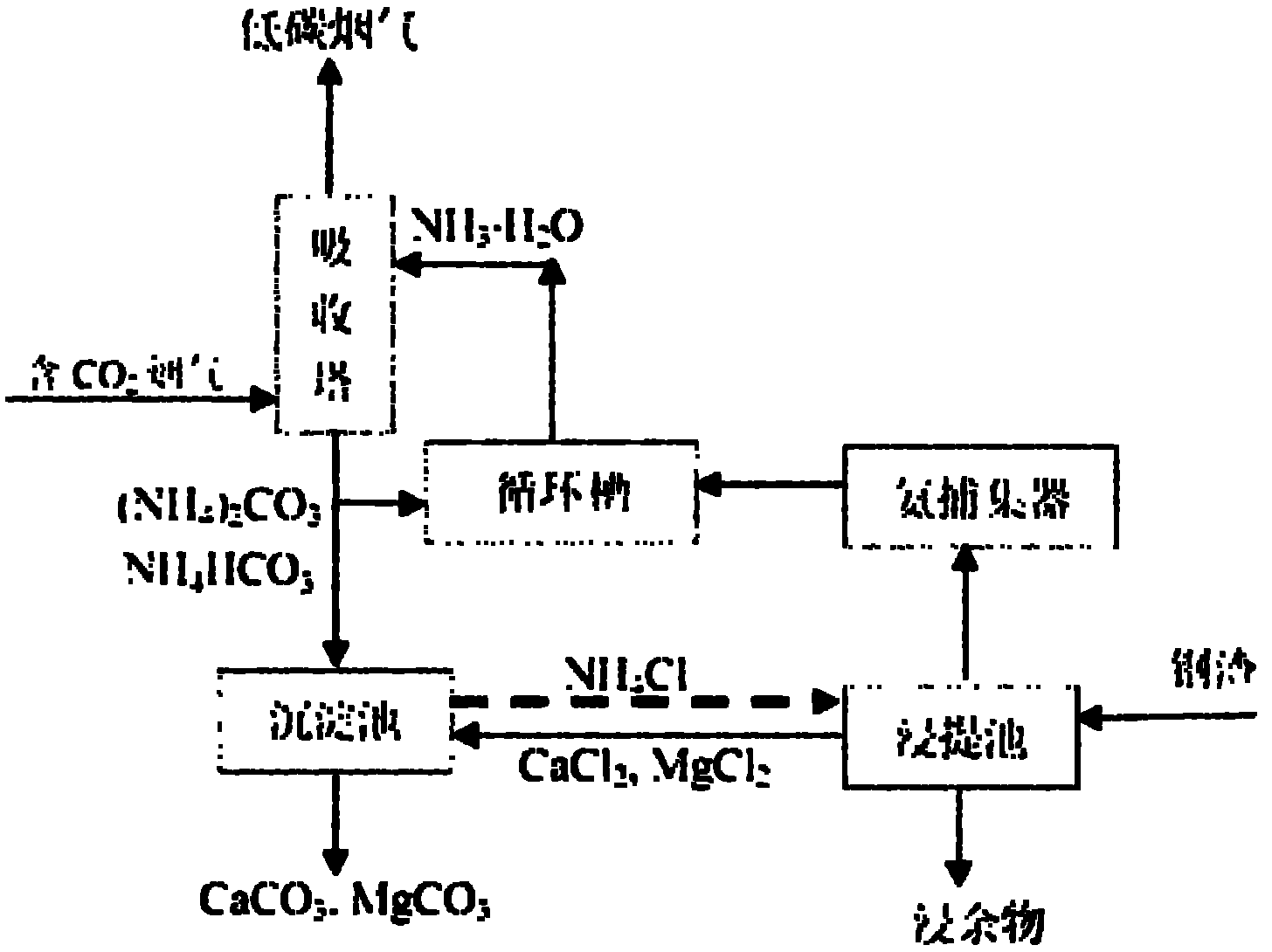
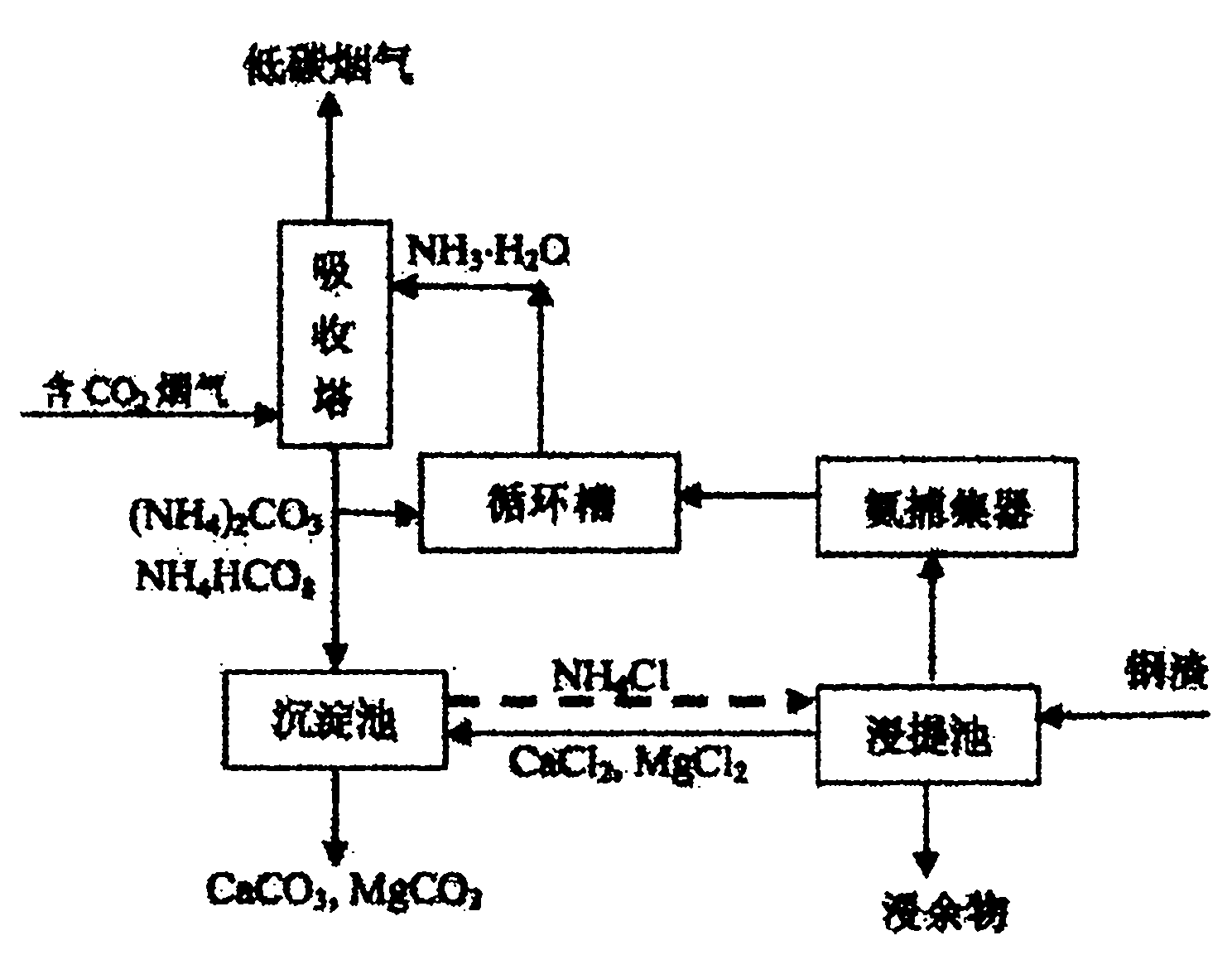
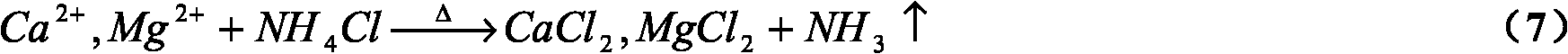Ammonia-chemical-chain-cycle-based carbon dioxide capture and conversion method
A carbon dioxide and chemical chain technology, applied in chemical instruments and methods, inorganic chemistry, separation methods, etc., can solve problems such as instability, unrecoverable carbonation products, large ammonia water, etc., and achieve the effect of saving costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Use air in the laboratory to prepare a continuous flow of mixed gas with a carbon dioxide concentration of 20%, and its flow rate is 2m 3 / h, use ammonia solution with a concentration of 10% as the carbon dioxide absorption liquid, and carry out gas-liquid countercurrent contact absorption in a packed tower with a diameter of 50mm, and the solution will gradually and finally be converted into a saturated ammonium bicarbonate solution, accompanied by a small amount of bicarbonate ammonium crystals.
[0036]Utilize 250mL of ammonium chloride solution with a concentration of 40%, put it into a 500mL ground-mouth conical flask, add 100g of blast furnace slag, wherein the calcium content (by weight of calcium oxide) is about 46%. Use a heater with magnetic stirring to heat while stirring, and finally control the leaching temperature at 90-100°C and control the pH in the range of 5.0-6.0. The overflowing ammonia gas is absorbed by a gas absorption bottle filled with water; A...
Embodiment 2
[0039] The capturing of carbon dioxide and the obtaining of ammonium bicarbonate solution are the same as in Example 1, except that the converter steel slag is used instead of the blast furnace water slag for the test. The calcium content of the converter slag used was about 37% by weight of calcium oxide.
[0040] Use 250mL of ammonium chloride solution with a concentration of 30%, put it into a 500mL conical flask, and add 120g of converter steel slag. Use a heater with magnetic stirring, stir while heating, and finally control the extraction temperature at 90-100°C, control the pH in the range of 5.5-6.5, and absorb the overflowing ammonia gas with a gas absorption bottle filled with water; After 1-2 hours of reaction, when the amount of ammonia finally released by the reaction system drops to a very low level, a certain amount of calcium hydroxide solution is also added to adjust the pH to the range of 8-10, so that the ammonia in the liquid phase is relatively low. Relea...
Embodiment 3
[0043] Absorption process is the same as embodiment 1, by controlling the absorption condition (raising the pH value of ammoniacal liquor), the decarburization product obtained by absorption is converted into ammonium carbonate; by implementing the method mentioned in embodiment 1) to the blast furnace slag The calcium and magnesium components are extracted to obtain the corresponding calcium chloride solution;
[0044] Then use ammonium carbonate solution and calcium chloride solution to react, the molar ratio of the two is 1:1, the final products are calcium carbonate and ammonium chloride, after filtration, the recovery rate of calcium is close to 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com