Bacillus amyloliquefaciens LC02 and application thereof
A technology of amylolytic spores and spore laccase, which is applied in the field of applied microorganisms, can solve the problem of less bacterial laccase, and achieve good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The property of embodiment 1 bacillus amyloliquefaciens LC02 spore laccase
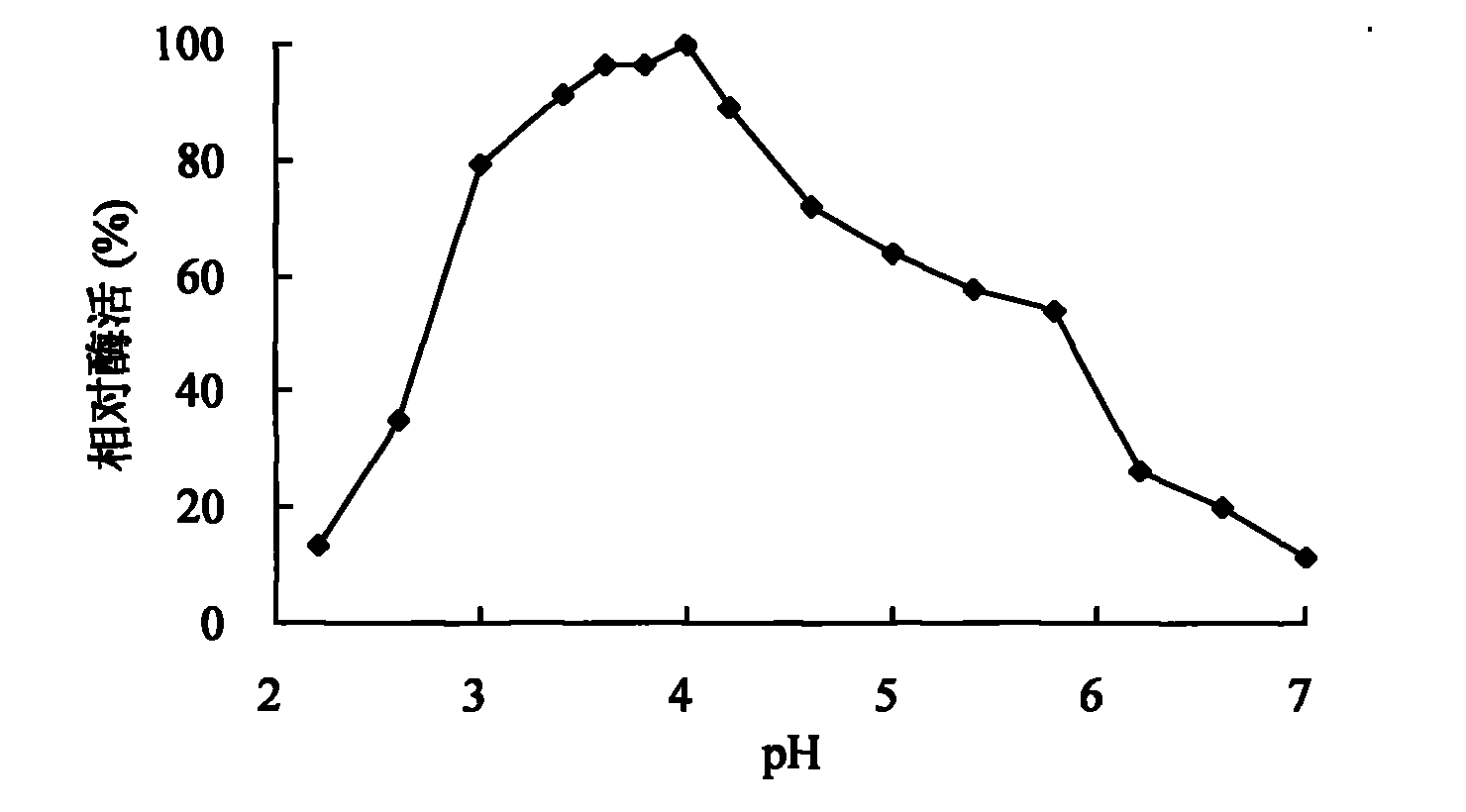
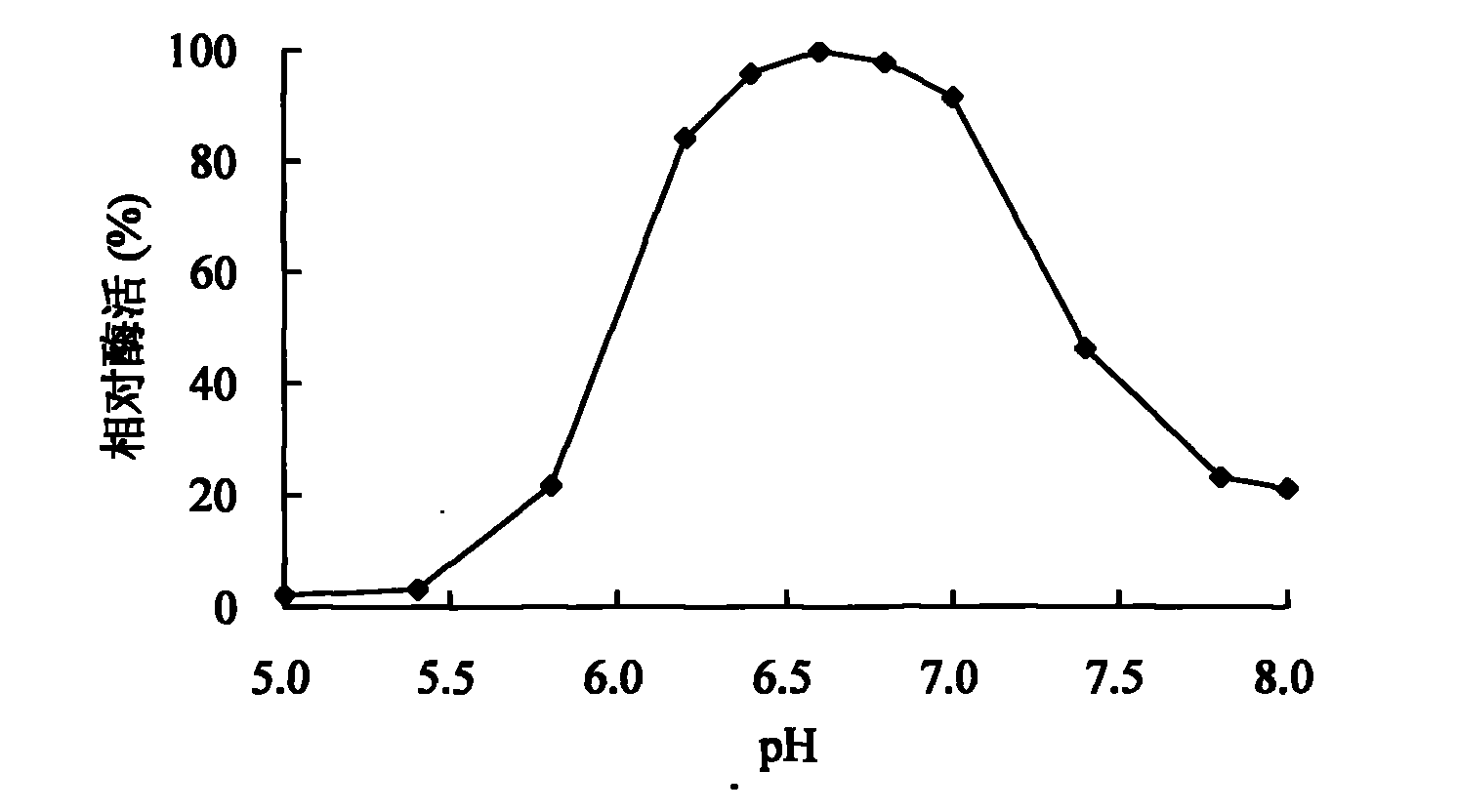
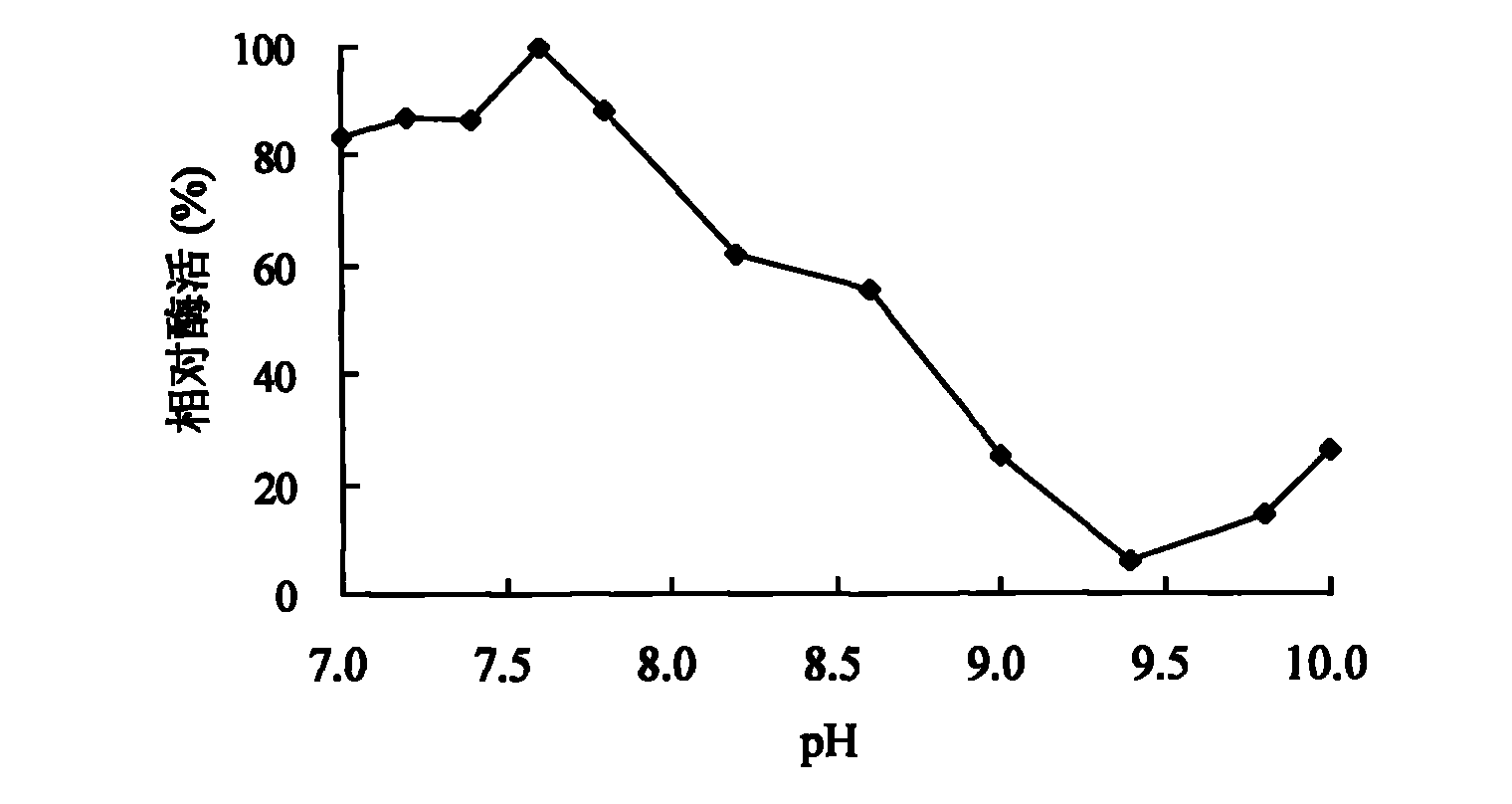
[0049] 1. Effect of pH value on activity and stability of spore laccase
[0050] The effect of pH on laccase activity using pH2.2-7.8 citric acid-phosphate buffer (0.1mol / L), pH7.4-9.0Tris-HCl (0.05mol / L) buffer and pH8.6-10.0 glycine -Sodium hydroxide buffer solution (0.05mol / L) is measured, and the influence of pH on the stability of laccase is by the citric acid-phosphate buffer solution of pH3.0,8.0, the Tris of pH9.0 by laccase at 30 ℃ -HCl buffer solution and pH 10.0 glycine-sodium hydroxide buffer solution were mixed and placed for 1-10 days to determine the remaining activity. Figure 1-3 It shows that the spore laccase provided by the invention has a wide range of catalysis, and can catalyze the substrate reaction in the scope of pH2.2-10.0. When using ABTS, syringaldazine and 2,6-dimethoxyphenol as substrates The measured pH optimums were 4.0, 6.6 and 7.6, respectively. Figure 4 T...
Embodiment 2
[0057] Example 2 Bacillus amyloliquefaciens LC02 spore laccase to the decolorization of synthetic dyes
[0058] 1. The reaction system of the decolorization experiment and the calculation of the decolorization rate
[0059] The experimental reaction system was 6ml, and pH 6.6 citric acid-phosphate buffer (0.1mol / L), dyestuff (see Table 1 for the species and final concentration), and Bacillus amyloliquefaciens LC02 spore laccase (final concentration of 1mg / ml) and acetosyringone (final concentration 0.1mmol / L). At the same time, the reaction system without adding Bacillus amyloliquefaciens LC02 spore laccase was used as a blank control. Samples were taken regularly, centrifuged at 12000 rpm for 2 minutes, and the absorbance values at the maximum absorption wavelengths (see Table 1) of each dye were measured. All measurements were repeated three times to obtain the average value. Then calculate the dye decolorization rate. The formula for calculating the decolorization rat...
Embodiment 3
[0065] Cloning of embodiment 3 Bacillus amyloliquefaciens LC02 spore laccase gene
[0066] 1. Cloning of strain laccase gene
[0067] Pick the above strains into 5mL LB liquid medium, culture overnight at 37°C, 200rpm. Genomic DNA of the above strains was extracted according to the instructions of the bacterial genome extraction kit from Omega Company, and detected by 1% agarose gel electrophoresis.
[0068] Genomic DNA was used as a template, and the upstream primer "CGG GAATTC GGCACTGGAAAAATTTGCAGATG" (restriction site EcoR I) and downstream primer "CCG CTCGAG TTACTGCTTATCCGTGACGTCC" (restriction site Xho I) amplifies the spore laccase gene of Bacillus amyloliquefaciens LC02.
[0069] Add the following reagents to a 200 μL PCR tube: ddH 2 O 13.75 μL, 10×Ex Taq Buffer 2 μL, 10 mmol / L dNTP mixture 1 μL, 10 μmol / L upstream primer 1 μL, 10 μmol / L downstream primer 1 μL, DNA template 1 μL, Ex Taq enzyme 0.25 μL, total volume 20 μL. A negative control was also set up. PCR ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com