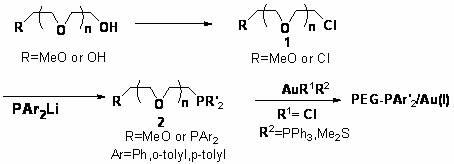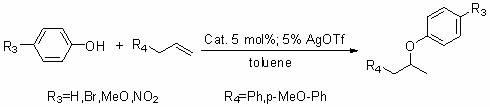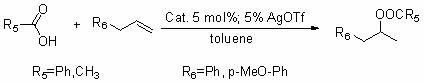Method for preparing supported gold catalyst and application thereof
A gold catalyst, supported technology, applied in the field of chemical catalytic materials and chemical applications, to achieve the effects of reducing pollution, promoting development, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] a. Preparation of chlorinated polyethylene glycol
[0034] Under the protection of argon, mix 2 mmol (10 g) linear polyethylene glycol monomethyl ether-5000 (MeO-PEG-5000) with 2 mmol (0.158 g) pyridine and 60 ml of anhydrous toluene and mix in half Add 0.236 g (2.8 mmol) of thionyl chloride dropwise within hours, stir and reflux for 5 h, add 30ml of dichloromethane (CH 2 Cl 2 ) And 2g of activated alumina were stirred for 2~3 times, 20min each time, then filtered and concentrated, then dropped into ether to precipitate out, and finally filtered and dried under vacuum at 30°C to obtain chloropolyethylene glycol (PEG- Cl) 9.32g, the yield is 90%.
[0035] b. Preparation of lithium diphenylphosphine
[0036] Under the protection of argon, mix 0.84g (120mmol) of lithium chips with 35ml of anhydrous tetrahydrofuran (THF), stir at 0℃ for 20min, and then slowly add 40mmol (8.8g) of diphenylphosphonium chloride and 15ml of anhydrous tetrahydrofuran (THF) mixed solution, stirred at ...
Embodiment 2
[0042] The supported gold catalyst of the present invention (PEG-PAr 2 / Au(I)) is used in the intramolecular cyclization reaction (Conia-Ene reaction) of 2-alkynyl-1,3-dicarbonyl compounds. The reaction formula is as follows:
[0043]
[0044] Specific operation steps: Under the protection of argon, mix 0.5 mmol 2-benzoyl-6-heptynoate ethyl ester with 2 ml anhydrous isopropanol and add 0.025 mol silver trifluoromethanesulfonate (AgOTf) and 0.025 mol supported gold catalyst (PEG-PPh 2 / Au(I)) for intramolecular cyclization reaction, the reaction temperature is 80℃, and TLC is used for tracking. After the reaction, the reaction liquid is dropped into ether to precipitate, then filtered, concentrated, and then obtained by column chromatography. The yield of benzoyl-2-methylenecyclopentanecarboxylic acid ethyl ester is 95%. The filter cake is drained by an oil pump, and the supported gold catalyst (PEG-PAr 2 / Au(I)) to be reused next time.
Embodiment 3
[0046] The supported gold catalyst of the present invention (PEG-PAr 2 / Au(I)) is used in the addition reaction between phenol and olefin molecules. The reaction formula is as follows:
[0047]
[0048] Specific operation steps: under the protection of argon, mix 0.5mmol of p-nitrophenol and 2mmol of p-methoxypropene, then add 0.025mol of silver trifluoromethanesulfonate (AgOTf), 0.025mol of supported gold catalyst (PEG- PAr 2 / Au(I)) and 5ml anhydrous toluene for the intermolecular addition reaction, followed by GC or TLC. After the reaction, the reaction solution was dropped into ether to precipitate, then filtered, concentrated, and then obtained by column chromatography 1-Methoxy-4-(2-(4-nitrophenoxy)propyl)benzene, the yield is 96%, the filter cake is drained by an oil pump, and the supported gold catalyst (PEG-PAr 2 / Au(I)) to be reused next time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com