Method for preparing insect repellant icaridin
A technology of icaridin and insect repellent, which is applied in the field of preparation of insect repellent, can solve the problems of increased preparation cost, inconvenient transportation, high purchase price, etc., achieves reduction of energy consumption and solvent loss, acceleration of reaction process, and reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0039] All reagents used in this example were purchased from Changzhou Chemical Reagent Co., Ltd., with a purity of ≥98%, and were not further processed before use.
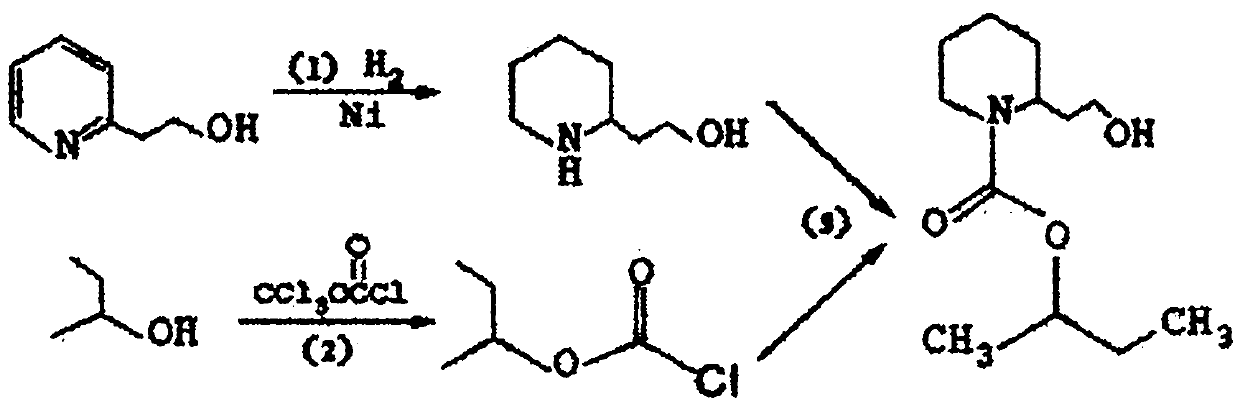
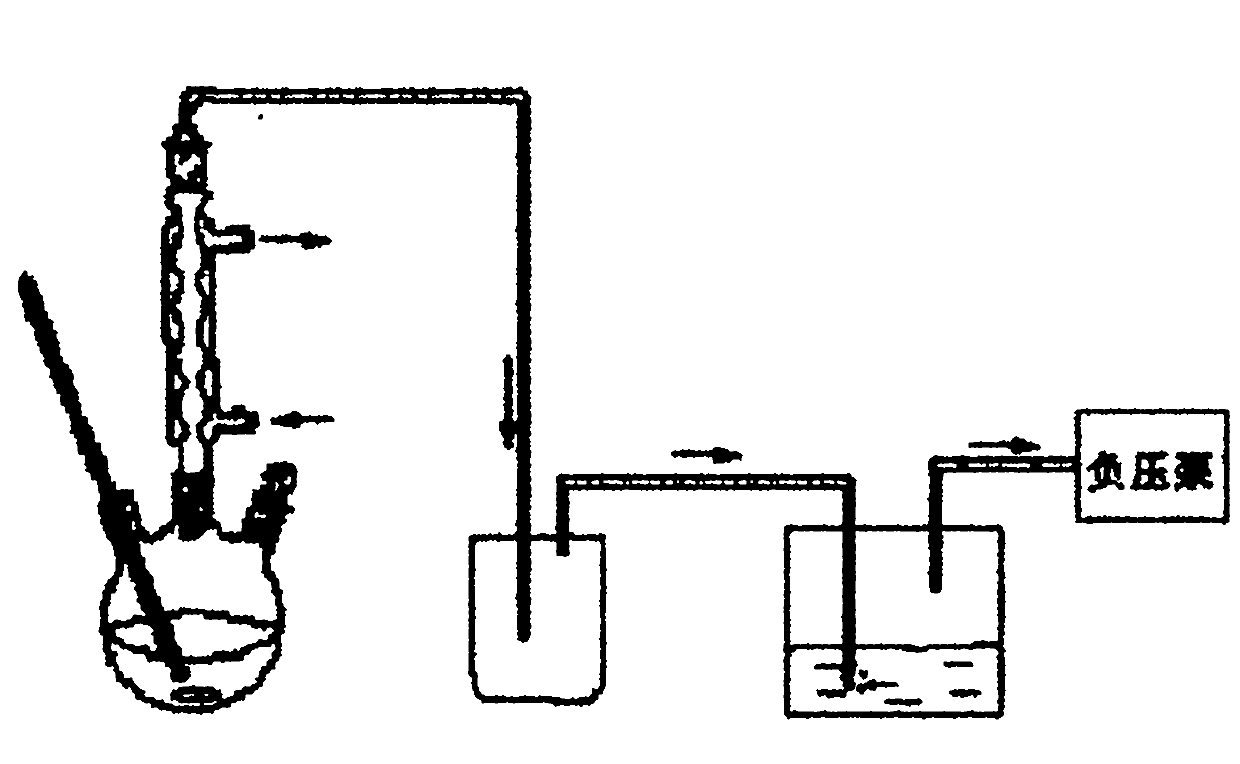
[0040] See figure 1 , the synthetic method of the present embodiment specifically comprises the following steps:
[0041] ① Preparation of 2-piperidine ethanol:
[0042] In a stirred 1L stainless steel autoclave, add 100g of 2-ethanolpyridine, 400g of solvent toluene and 5g of catalyst Raney nickel (SC-4100), close the autoclave, replace the air in the autoclave with nitrogen three times, and then use hydrogen Replace the gas in the reactor twice; then fill in hydrogen to make the reaction pressure in the reactor reach 4MPa, and slowly raise the temperature to 60°C under stirring, so that one of the products is a catalytic hydrogenation reaction of 2-piperidine ethanol, continue After stirring and reacting for 6 hours, a small amount of reaction liquid in the reactor was taken for detection, and gas chromatogra...
Embodiment 2)
[0076] All the other synthetic methods of the present embodiment are the same as in Example 1, except that:
[0077] In step 2., also comprise the purifying operation of sec-butyl chloroformate, after organic phase filtration removes desiccant anhydrous sodium sulfate, the solvent dichloromethane in the filtrate is evaporated earlier and reclaims mechanically, then remaining organic matter underpressure distillation, The fraction at 148-152°C / 5mmHg was collected to obtain 182 g of sec-butyl chloroformate with a yield of 91%. The purity of sec-butyl chloroformate was 98.4% as detected by GC.
[0078] In step ③, when synthesizing icaridin, the amount of solvent methylene chloride added is 40L, and the temperature in the reaction kettle drops to 0°C, and 14.0kg of sec-butyl chloroformate refined according to the method of step ② is added dropwise The reacted mixed material was washed with purified water twice and washed with saturated brine once, dried over anhydrous sodium sulfa...
Embodiment 3)
[0081] All the other synthetic methods of the present embodiment are the same as in Example 1, except that:
[0082] In step ②, the source material of chloroformic acid group is bis(trichloromethyl)carbonate. The chemical formula of bis(trichloromethyl)carbonate is Cl 3 COCO 2 CCl 3 , also known as triphosgene or solid phosgene, is a white crystal with a phosgene-like odor. One molecule of triphosgene can be decomposed into three molecules of phosgene. When triphosgene reacts with sec-butanol to prepare sec-butyl chloroformate, it first becomes phosgene, and the phosgene just generated reacts with sec-butanol immediately to generate hydrogen chloride gas and sec-butyl chloroformate, and the reaction formula is as follows:
[0083]
[0084] During preparation, under good ventilation conditions, add 112g (1.5mol) of sec-butanol, 500mL of solvent methylene chloride, 118g of catalyst pyridine and 150g (0.5mol) of bis(trichloromethyl) carbonate In the 2L three-necked bottle...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Injection volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com