Method for preparing penicillin binding protein combined with beta-lactam antibiotic and special recombinant bacterium
A technology of binding protein and recombinant bacteria, which is applied in the field of penicillin binding protein, can solve the problem of low efficiency of penicillin binding protein, and achieve the effect of high yield and low solution yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of recombinant bacteria
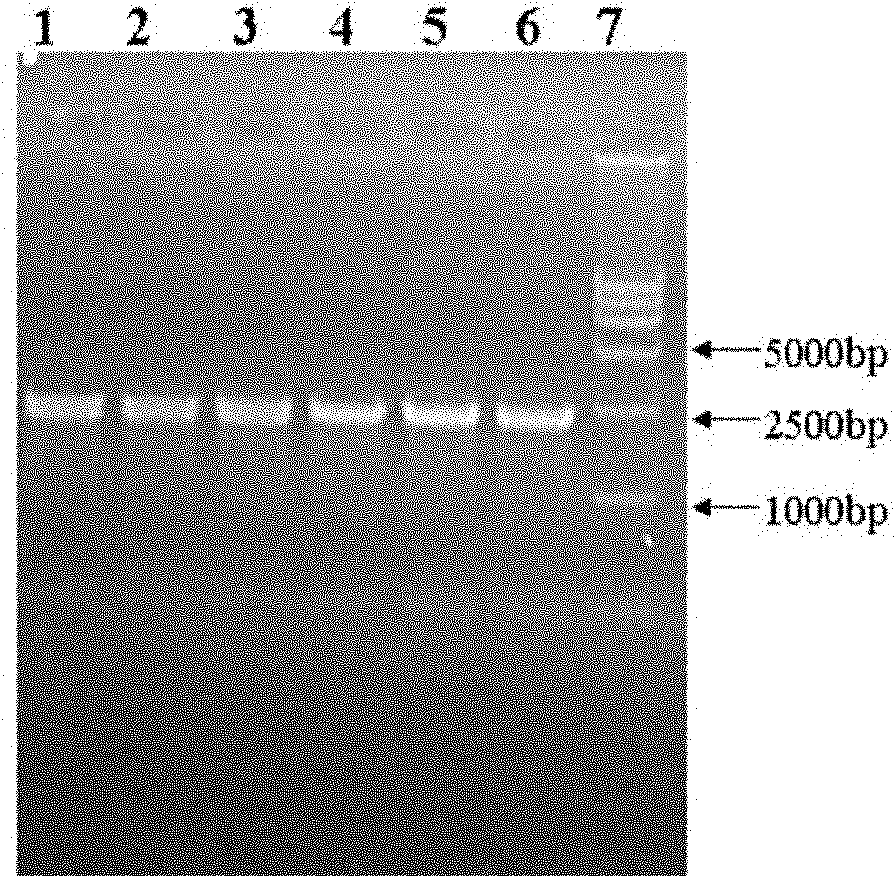
[0031] The Streptococcus pneumoniae R6 genome was used as a template, and the following primers were used for PCR amplification to obtain PCR products; the PCR products were digested with restriction endonucleases NdeI and XhoI, and the PCR products were subjected to 1% agarose gel electrophoresis to recover about 2250bp Target gene fragment (i.e. PBP2x target gene); electrophoresis results are as follows figure 1 As shown, lanes 1-6 are the target genes, and lane 7 is the marker.
[0032] Upstream primer: 5'-TT CATATG ATGAAGTGGACAAAAAGA-3' (5' end contains NdeI restriction site and protective base)
[0033] Downstream primer: 5'-TT CTCGAG TTAGTCTCCTAAAGTTAAT-3' (3' end contains XhoI restriction site and protective base)
[0034] Recover the target gene fragment, connect it to the PMD19-T vector, transform the ligated product into Escherichia coli DH5α, screen the positive single clone through resistance, cul...
Embodiment 2
[0038] Embodiment 2, preparation and purification of protein
[0039] 1. Protein preparation
[0040] Inoculate the recombinant strain BL21-pET28b-PBP2x into LB liquid medium containing 30ug / mL kanamycin, shake at 250rpm at 37°C, and culture overnight (12h) to obtain seed liquid; take 1mL seed liquid and inoculate 100mL kanamycin-containing Namycin 30ug / mL LB liquid medium, shake at 250rpm at 37°C, to the A of the culture system 600 When it is 0.6, take out 1mL of the bacterial liquid as the uninduced control, add IPTG to the rest of the bacterial liquid to a final concentration of 1mM, shake the bacteria at 200pm 30°C, and start from the 3rd hour of induction (recorded as the 0th hour when adding IPTG), every Take out 1mL of bacterial liquid every hour, and the induction time is 3h, 4h, 5h, 6h, 7h, 8h and overnight (12h); centrifuge the collected bacterial liquid at 10000rpm at 4°C for 5min to collect the bacterial cells; use Tris-HCL (10mM Tris pH7.0) to resuspend the bact...
Embodiment 3
[0064] Embodiment 3, protein activity detection
[0065] 1. ELISA plate coated with penicillin-binding protein: the penicillin-binding protein prepared in Example 2 is configured with coating buffer to 1 ug / mL; 100 uL per well.
[0066] 2. Enzyme labeling substance: HRP-labeled ampicillin, its working concentration is 1ug / mL, and the working solution of the enzyme labeling substance is obtained by diluting the enzyme labeling substance with the sample diluent.
[0067] 3. Standard product of β-lactam antibiotics: the standard product is penicillin G freeze-dried powder, which was purchased from Sigma-Aldrich Company in the United States; the product catalog number is 46616; the penicillin G was dissolved in the sample diluent to obtain the following different concentrations Standard solution: 8.1μg / L, 2.7μg / L, 0.9μg / L, 0.3μg / L, 0.1μg / L.
[0068] 4. Substrate chromogenic solution: composed of liquid A and liquid B, liquid A is an aqueous solution of 2% carbamide peroxide, liqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com