Preparation method of 4-nitrophenyl sodium phosphate salts
A technology of sodium nitrophenyl phosphate and p-nitrophenol, which is applied in the field of organic synthesis, and can solve problems such as the impact on the environment and the personal safety of experimental operators, the difficulty in meeting the quality requirements of biochemical and diagnostic reagents, and the unavoidable pollution of heavy metal barium ions , to achieve the effect of improving product purity, reducing the residue of heavy metal element barium, and simplifying the process implementation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
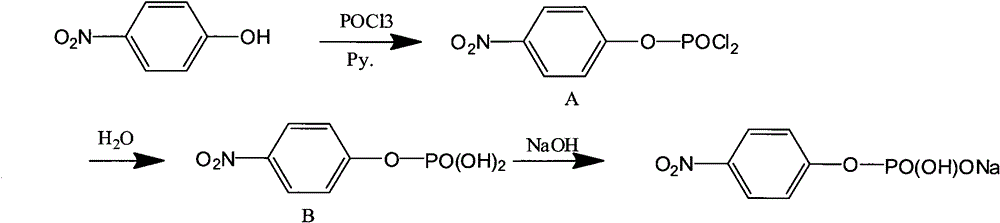
[0026] The process step in the following embodiment 1 is with reference to appendix figure 1 The reaction scheme in .
[0027] 1. Synthesis and separation of intermediate product A:
[0028] Add p-nitrophenol (pNP) and pyridine (Py.) in a molar ratio of 1:6 into a three-necked flask, and stir to dissolve. Then control the temperature at 10-15°C, and after the temperature stabilizes within this range, use a dropper to pour the refined phosphorus oxychloride (POCl 3 ) slowly dropwise into the three-necked flask, after adding one molar equivalent, add an appropriate amount of POCl 3 , to avoid the generation of disubstituted and trisubstituted by-products, and then continue to stir at this temperature for 10 hours to obtain a slurry. At this time, TLC (petroleum ether: ethyl acetate = 1:3) showed that no starting material p-nitrophenol (pNP) remained.
[0029] Suction filter the slurry, the filter cake is washed with toluene, and the obtained filtrate is distilled off under r...
Embodiment 2
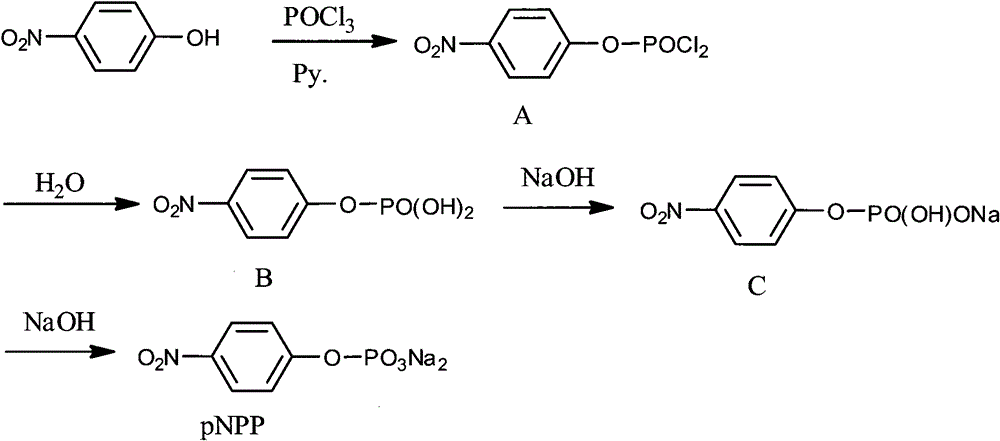
[0041] The processing steps in the following embodiment 2 all refer to the appended figure 2 The reaction scheme in .
[0042] 1. Synthesis and separation of intermediate product A:
[0043]Add p-nitrophenol (pNP) and pyridine (Py.) in a molar ratio of 1:8 into a three-neck flask, and stir to dissolve. Then control the temperature at 10-15°C, and after the temperature stabilizes within this range, use a dropper to pour the refined phosphorus oxychloride (POCl 3 ) slowly dropwise into the three-necked flask, after adding one molar equivalent, add an appropriate amount of POCl 3 , to avoid the generation of disubstituted and trisubstituted by-products, and then continue to stir at this temperature for 6-10 hours to obtain a slurry. At this time, TLC (petroleum ether: ethyl acetate = 1:3) showed that no starting material p-nitrophenol (pNP) remained.
[0044] Suction filter the slurry, the filter cake is washed with toluene, and the obtained filtrate is distilled off under r...
Embodiment 3
[0063] 1. Synthesis and separation of intermediate product A:
[0064] Add p-nitrophenol (pNP) and pyridine (Py.) in a molar ratio of 1:2 into a three-necked flask, and stir to dissolve. Then control the temperature at 10-15°C, and after the temperature stabilizes within this range, use a dropper to pour the refined phosphorus oxybromide (POBr 3 ) is slowly added dropwise to the three-necked flask, after adding one molar equivalent, add an appropriate amount of POBr 3 , to avoid the generation of disubstituted and trisubstituted by-products, and then continue to stir at this temperature for 6-10 hours to obtain a slurry. At this time, TLC (petroleum ether: ethyl acetate = 1:3) showed that no starting material p-nitrophenol (pNP) remained.
[0065] Suction filtration slurry, filter cake is washed with toluene, the filtrate decompression distillation that obtains is sloughed off toluene, excessive phosphorus oxybromide (POBr 3 ) and pyridine (Py.) were sealed and stored to pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com