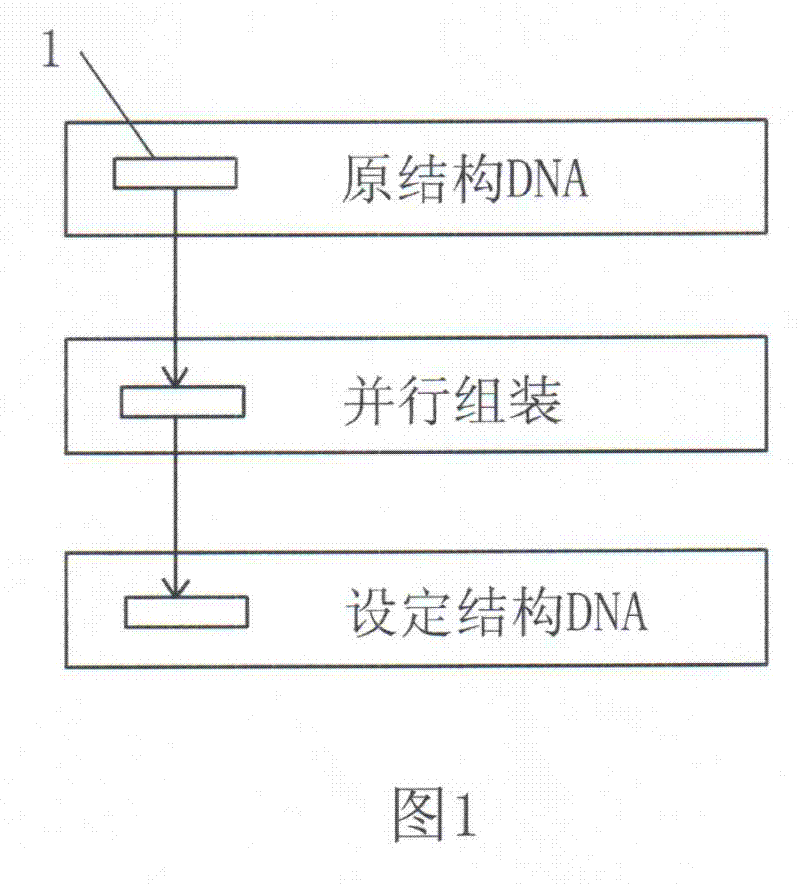
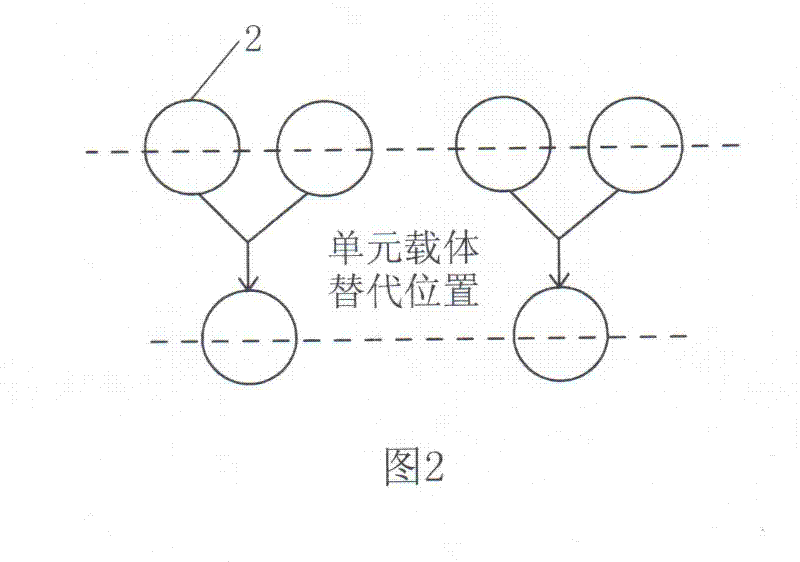
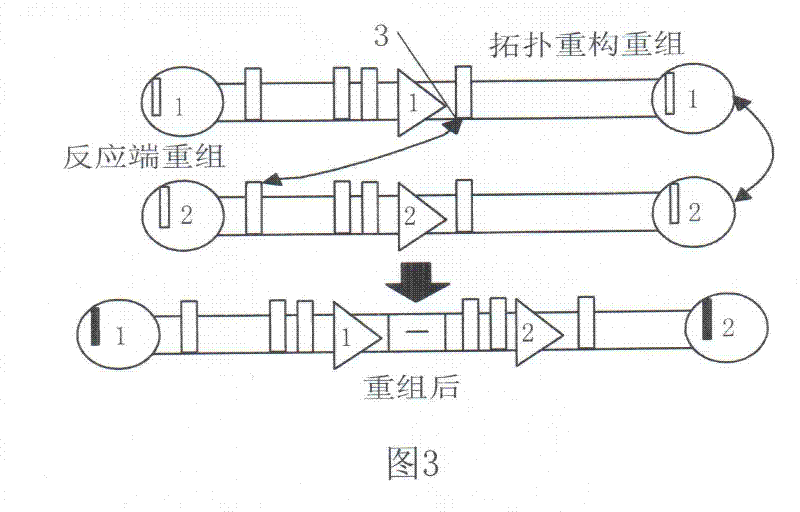
A Method for Parallel Assembly of Multiple Segments of DNA
A DNA sequence and fragment technology, applied in the field of genetic engineering, can solve the problems of low recombination efficiency, difficult in vitro operation of long DNA fragments, and difficulty in parallel operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0343] Example 1: Assemble luxA, luxB, luxC, luxD, luxE using a circular assembly system.
[0344] Original plasmid: pAH57, expressing Lambda Int Xis. pAH69, expresses HK022 Int. pAH129, expresses Phi80 Int Xis. pAH83 expresses HK022 Int Xis. pAH70, containing HK022 attP. Retrieved from CGSC (http: / / cgsc.biology.yale.edu / ). pCMR expresses HK022-Lambda Chemira Int Xis. pAH69Pir, expresses HK022 Int and Pir genes. pCMRPir expresses HK022-Lambda Chemira Int Xis and Pir genes. pAH129E, obtained by destroying lambda C1 in pAH129 with HindIII.
[0345] Construct the plasmid:
[0346] (1) Construction of auxiliary plasmids:
[0347] Synthetic primers:
[0348] EVF: ACCTGACCGCTATCCCTGA
[0349] EVR: GCCCTTCAATCGCCAGA
[0350] IHF: TTGACTATTTTACCCTCTGGCGG
[0351] IHR:TCCGACTTATGCCCGAGAAGACGTTG
[0352] IIF: CAACGTCTTCTCGGGCATAAGTCGGA
[0353] IIR: AATAACATGTAGCTTGGCATTGCTTATCAA
[0354] Synthetic gene:
[0355] p1pir:ACTAGTTTGAATTGGTCACGACTTTGCGAAGCAAAGTCTAGTGAGTATACT...
Embodiment 2
[0490] Embodiment 2: luxA, luxB, luxC, luxD, luxE are assembled using a linear assembly system.
[0491] (1) Build pLUHelp:
[0492] Gene synthesis:
[0493] CrossInt:[AY048721.1:878-1258][AY048721.1:1259-1602][AY048715.1:1614-2236]TTGGCACTGGCTGATCAGCTAGCACATGT
[0494] Experimental steps:
[0495] Construction of pLUHelpH: digest pAH83 with restriction endonucleases NcoI and EcoRI, separate the 3822bp fragment in the digested product by agarose gel electrophoresis, treat the separated product with alkaline phosphatase, and label the product as cAH83. CrossInt was digested with restriction endonucleases EcoRI and PciI, and a 1375bp fragment in the digested product was separated by agarose gel electrophoresis, labeled as cCrossInt. Ligate cCrossInt and cAH83 with T4 DNA ligase, electrotransform the ligated product into a competent E. coli DH5α, spread the transformed product on an LB agar plate containing ampicillin, and incubate at 30°C until visible For single clones, pick ...
Embodiment 3
[0582] Example 3: Assembly of the lux gene in a circular host-free recycling system.
[0583] (1) Build pUC-Gate:
[0584] Synthetic primers:
[0585] PG1:CGTTCGCAGAATTGGGAATC
[0586] PG2: GGGATAGCAAGCCCAATAGG
[0587] M13F:TGTAAAACGACGGCCAGT
[0588] M13R: CAGGAAACAGCTATGACC
[0589] Synthetic Gene:
[0590] Gate(克隆在pQLV中):AAGCTTGCGGCCGCGAAGTTCCTATTCCGAAGTTCCTATTCTCTAGAAAGTATAGGAACTTCTTAATTAACCAGCCTCGCAGAGCAGGATTCCCGTTGAGCACCGCCAGGTGCGAATAAGGGACAGTGAAGAAGGAACACCCGCTCGCGGGTGGGCCTACTTCACCTATCGGTACCTTCGAGAGCTCCACCGAGTGACTAGTATGATGAATTCCACACGGTGGTCGACTACGTGCTAGCCTCGAGCAATTG
[0591] Experimental procedure: PCR was performed on Gate with GP1 and PG2, and the obtained fragment was digested in Fermentas T Buffer with MfeI and HindIII. Enzyme digestion product solution recovery. Labeled fragment cGate. The pUC18 vector was digested with EcoRI and HindIII in Fermentas R Buffer, and FastAP was added for dephosphorylation. Enzyme digestion product solution recovery. Labeled a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com