Pyridostigmine bromide odor masking orally disintegrating tablets and preparation method thereof
A technology of pyridostigmine bromide and orally disintegrating tablets, which is applied in the field of new preparations, can solve the problems of inconvenient long-term application of injections, inconvenient transportation and carrying of syrups, slow disintegration of sugar-coated tablets, etc., and shorten the disintegration time , The taste is cool and slightly sweet, and the taste is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The weight composition ratio of each component contained in the prescription is:
[0031] 7.5 parts of pyridostigmine bromide
[0032] Eudragit E100 7.5 servings
[0033] Mannitol 50 parts
[0034]15 parts microcrystalline cellulose
[0035] Cross-linked polyvinylpyrrolidone 5 parts
[0036] Aspartame 2.5 parts
[0037] Magnesium stearate 5 parts
[0038] Preparation:
[0039] Pass pyridostigmine bromide through 80-mesh sieve, add to molten Eudragit E100, mix well, rapidly cool the eutectic, pulverize, sieve 80-100-mesh powder, add mannitol and other auxiliary materials, mix evenly, and directly compress the powder into tablets 1000 orally disintegrating tablets with a tablet weight of 200 mg were obtained.
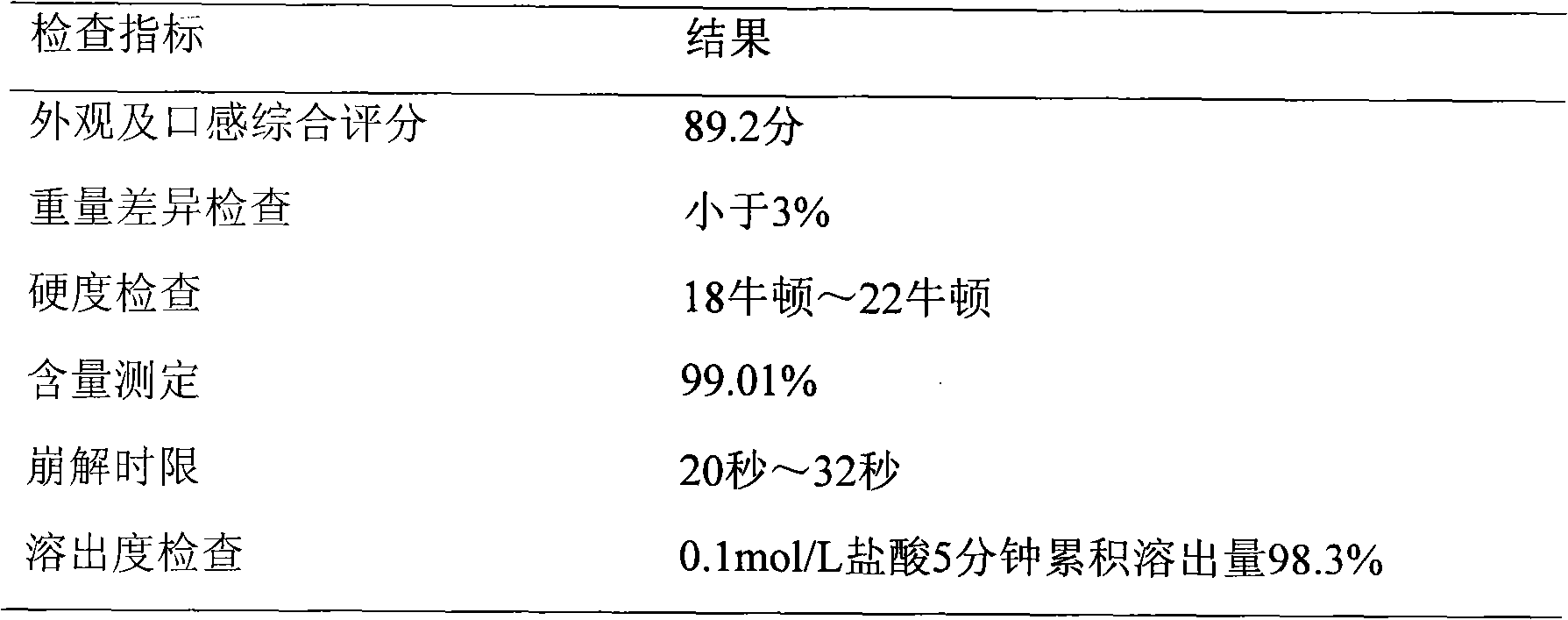
[0040] test results:
[0041]
Embodiment 2
[0043] The weight composition ratio of each component contained in the prescription is:
[0044] 7.5 parts of pyridostigmine bromide
[0045] Eudragit E100 30 servings
[0046] Lactose 17.5 parts
[0047] Sorbitol 20 parts
[0048] Sodium carboxymethyl starch 15 parts
[0049] Low-substituted hydroxypropyl cellulose 5 parts
[0050] Acesulfame K 1.5 parts
[0051] 1.5 parts mint essence
[0053] Preparation:
[0054] Pass pyridostigmine bromide through 80 mesh sieve, add to molten Eudragit E100, mix well, rapidly cool the eutectic, pulverize, screen 80-100 mesh powder, add lactose and other auxiliary materials (except talc powder and mint essence), mix Homogenize, make soft material with distilled water, granulate with 24-mesh sieve, dry at 40°C, granulate with 20-mesh sieve, add peppermint essence and talcum powder, mix and compress to obtain 1000 orally disintegrating tablets with a tablet weight of 200 mg.
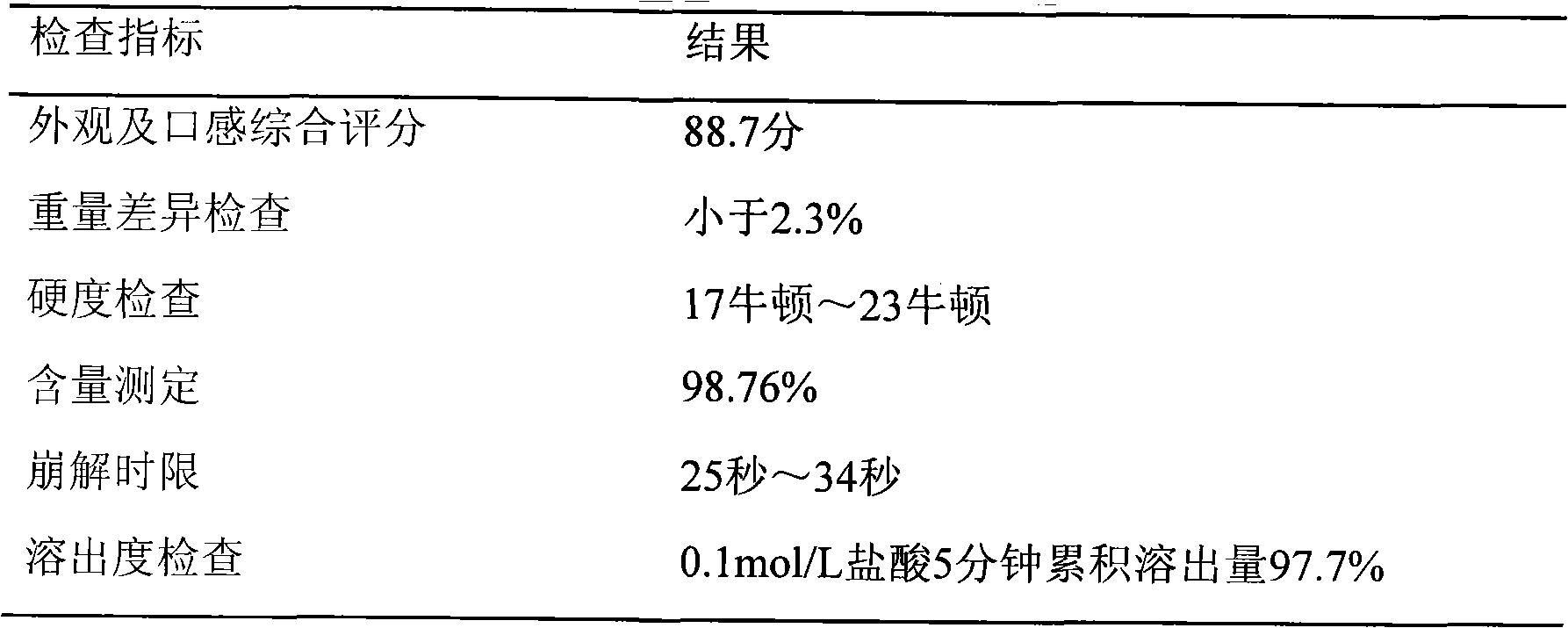
[0055] test results:
[005...
Embodiment 3
[0058] The weight composition ratio of each component contained in the prescription is:
[0059] Pyridostigmine bromide 15 parts
[0060] Eudragit E100 25 servings
[0061] Lactose 30 parts
[0062] Mannitol 10 parts
[0063] Sodium carboxymethyl starch 8 parts
[0064] Croscarmellose sodium 12 parts
[0065] Sucralose 1.6 parts
[0066] Magnesium stearate 2.4 parts
[0067] Preparation:
[0068] Take Eudragit E100, dissolve 90 parts of ethanol solution, add pyridostigmine bromide and mix evenly, evaporate most of the solvent, dry under vacuum and reduce pressure, pulverize, screen the 80-100 mesh powder, add lactose and other auxiliary materials, mix evenly, and press the powder directly 1000 orally disintegrating tablets with a tablet weight of 250 mg were obtained.
[0069] test results:
[0070]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com