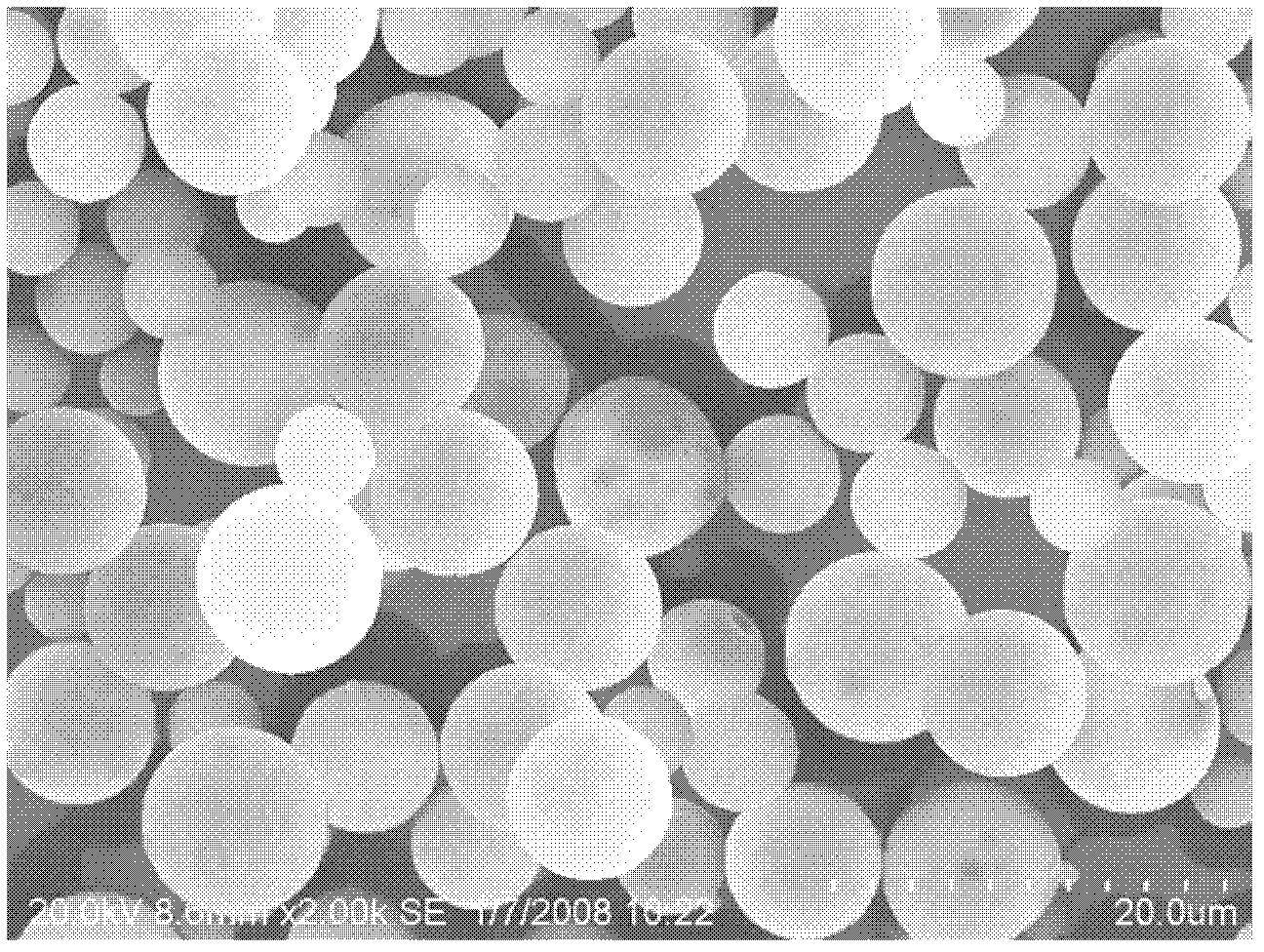Preparation method of PLGA swine influenza DNA vaccine microsphere
A technology of DNA vaccine and swine flu, which is applied in the field of preparation of DNA vaccine microspheres, can solve the problems of short duration and easy degradation of swine flu DNA vaccine, prolong the time of drug action, and the preparation method is simple, easy, and highly efficient. The effect of encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0018] Specific embodiment one: the preparation method of the PLGA porcine influenza DNA vaccine microsphere of the present embodiment is prepared according to the following steps:
[0019] 1. Weigh 25mg of PLGA and dissolve it in 0.75mL of dichloromethane. After it is completely dissolved, add 40μl of swine influenza virus H3N2 subtype HA gene recombinant plasmid DNA solution, and ultrasonic emulsify for 10s under ice bath conditions to obtain colostrum. Add 20 mL of polyvinyl alcohol with a mass concentration of 2% at a rotating speed of 7000 r / min to obtain double emulsion;
[0020] 2. Magnetically stir the double emulsion for 5 hours at a speed of 200r / min, then centrifuge at a speed of 4000r / min for 10 minutes, collect the microspheres and wash them with sterilized water for 3 times, and then place them at -10~-50℃ Vacuum freeze-drying under the condition of PLGA porcine influenza DNA vaccine microspheres is completed; wherein step 1 has 2 mg of porcine influenza virus H3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com