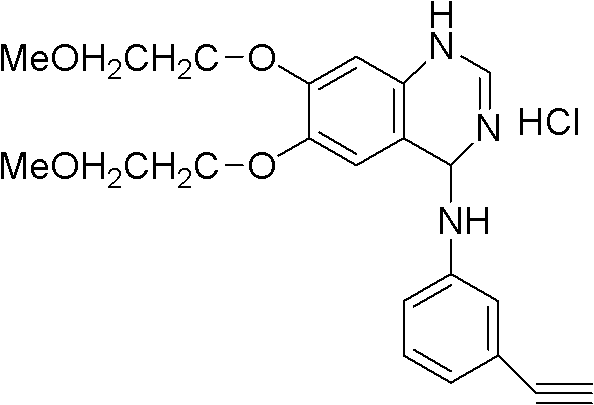
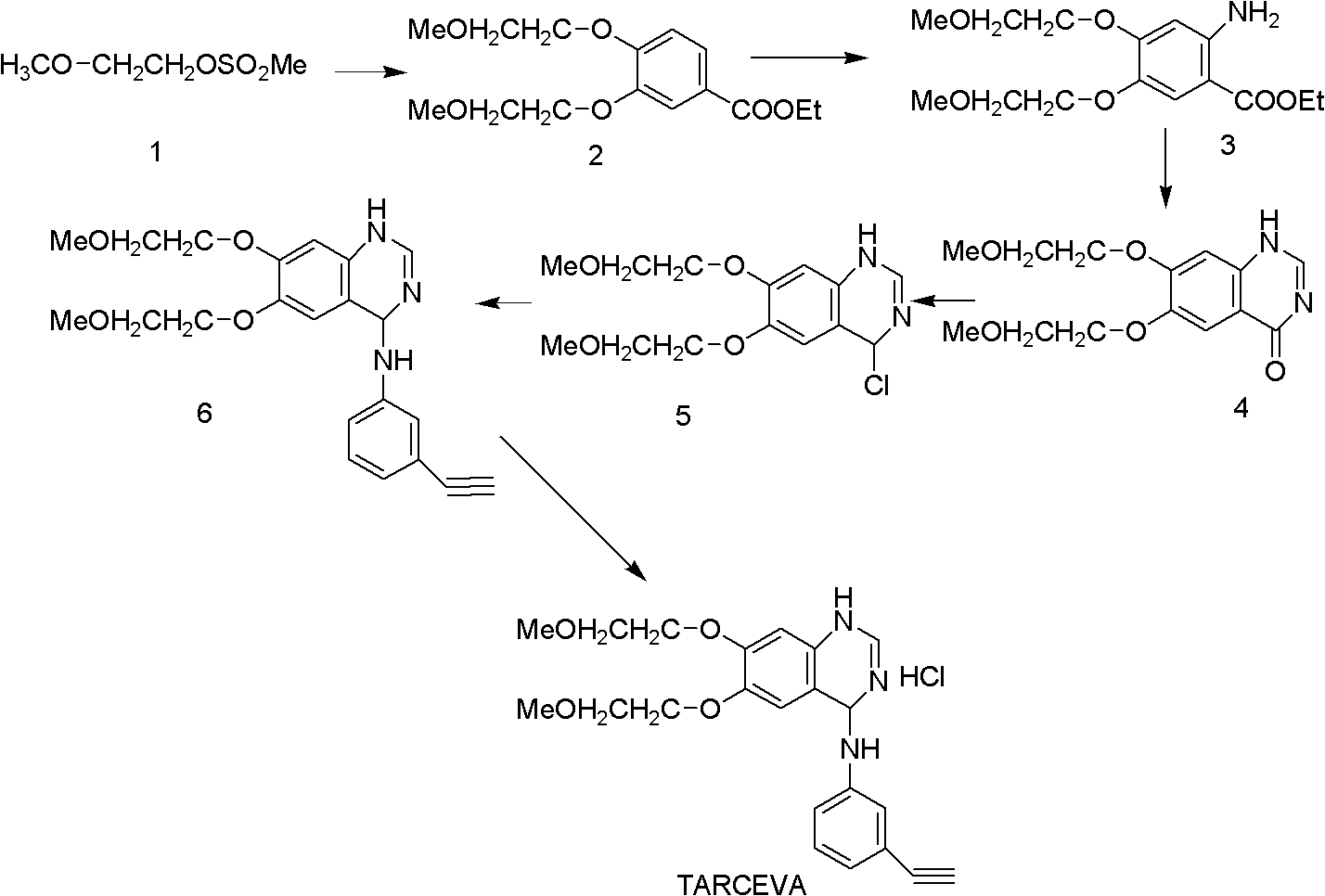
Preparation method of tarceva
A methoxyethoxy and intermediate technology, applied in the field of preparation of tasewa, can solve the problems of carbonization and deterioration of reactants, incomplete or polynitration, uncontrollable temperature, etc., and achieves mild conditions, high selectivity, high The effect of easy large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the preparation of intermediate 1 methanesulfonic acid (2-methoxyethyl ester)
[0033]Add 0.25L ethylene glycol monomethyl ether and 0.25L triethylamine into the reaction flask, and add 0.20L p-methanesulfonyl chloride in batches under ice-salt bath cooling, keeping the internal temperature not exceeding 5°C. After the addition, keep warm at 5-10°C for 5 hours, filter, slowly pour the reaction solution into 2.5L ice water for dilution, extract 3 times with ethyl acetate (1.0L×3), combine the organic phases, and dilute with 3% dilute hydrochloric acid (v / v) wash 3 times (0.3L×3), wash once with water (0.3L), wash once with saturated sodium chloride solution (0.3L), and dry over anhydrous sodium sulfate. The desiccant was filtered off, concentrated and evaporated to remove the solvent to obtain a white transparent oily liquid, which was identified as methanesulfonic acid (2-methoxyethyl ester) (intermediate 1) by NMR H spectrum, yield: 98%, detected by HPLC,...
Embodiment 2
[0034] Embodiment 2, intermediate 23, the preparation of 4-two (2-methoxyethoxy) ethyl benzoate
[0035] In a three-necked flask with mechanical stirring, add 18.2g of ethyl 3,4-dihydroxybenzoate (0.1mol), 110ml of water, 100ml of DMF and 30g of potassium carbonate, heat to 90-95°C, and dropwise add 38g of intermediate Compound 1 methanesulfonic acid (2-methoxyethyl ester) (0.25mol), after the addition was complete, the mixture was stirred for another 4 hours and 30 minutes, and stopped. The reaction solution was slowly added dropwise to 800ml of water under stirring to obtain a relatively dispersed solid, which was dried to obtain 228.4g of an intermediate with a yield of 95%. The purity was 99.2% by HPLC detection.
Embodiment 3
[0036] Embodiment 3, intermediate 33, the preparation of 4-bis(2-methoxyethoxy)-5-aminobenzoic acid ethyl ester
[0037] Add 25.7g (86mmol) intermediate 23,4-bis(2-methoxyethoxy)ethyl benzoate and 100ml ethyl acetate to the reaction flask, cool to 0°C in an ice-salt bath and slowly add 200ml Containing the ethyl acetate solution of 34ml fuming nitric acid, after the dropwise addition is completed, the stirring reaction was continued for about 1 hour. After the reaction was completed, 400ml of water was added to dilute, the layers were separated, and the lower layer was extracted with 50ml×3 ethyl acetate, and the organic layers were combined. Wash twice with water (50ml×2), and dry over anhydrous magnesium sulfate. The solvent was removed by rotary evaporation to obtain an oily substance. The resulting oil was added to the reaction kettle, and then 250ml of absolute ethanol and 0.5g of 5% Pd / C catalyst were added. Under the protection of nitrogen, hydrogen gas was introduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com