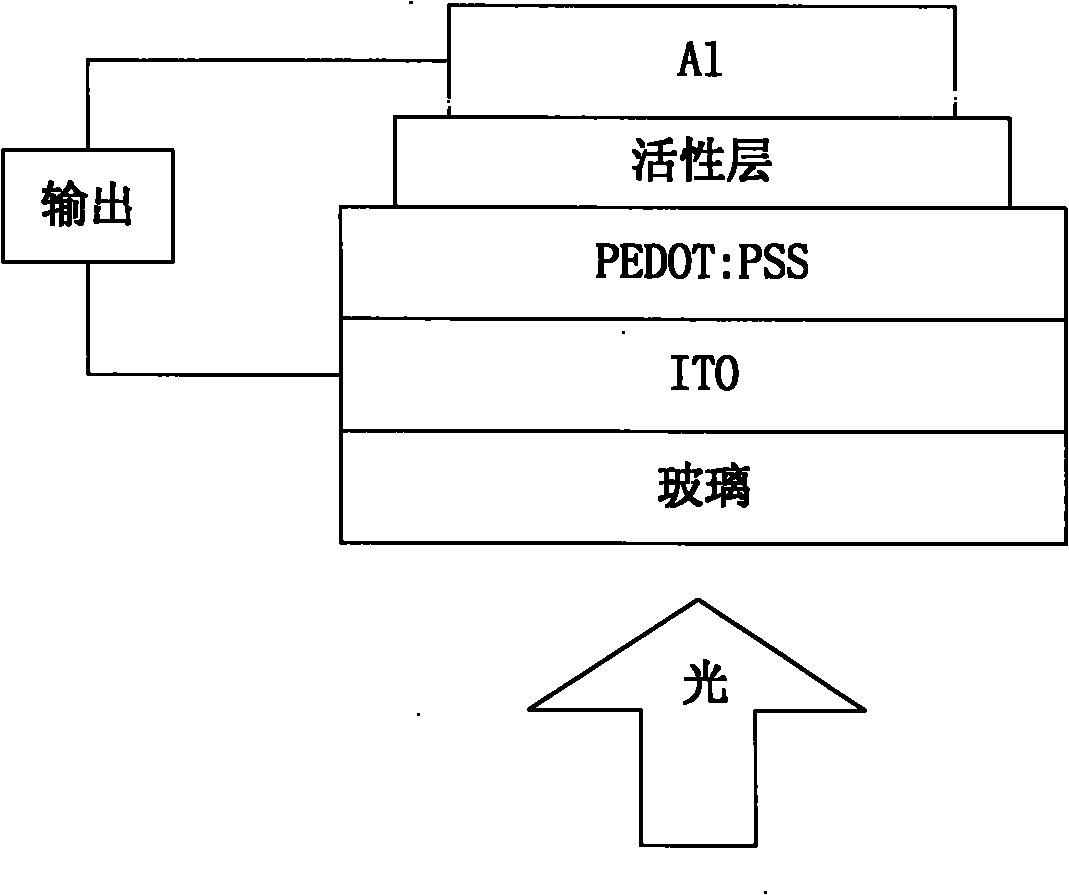
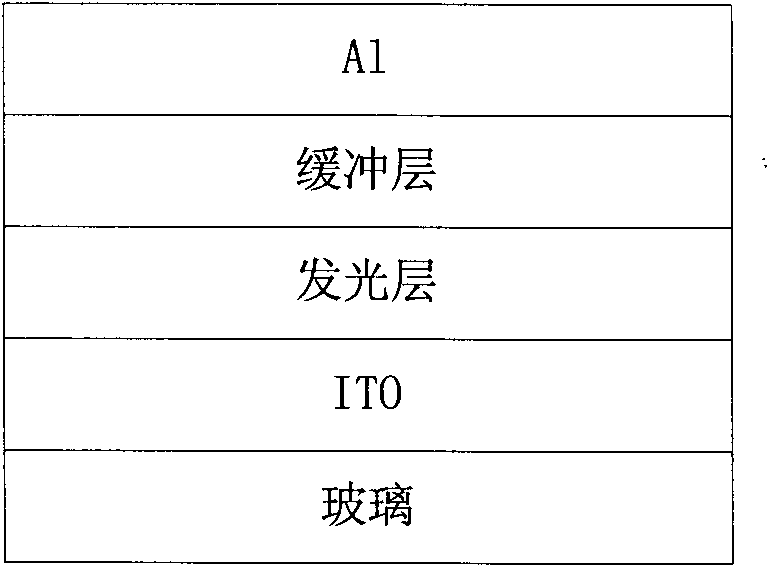
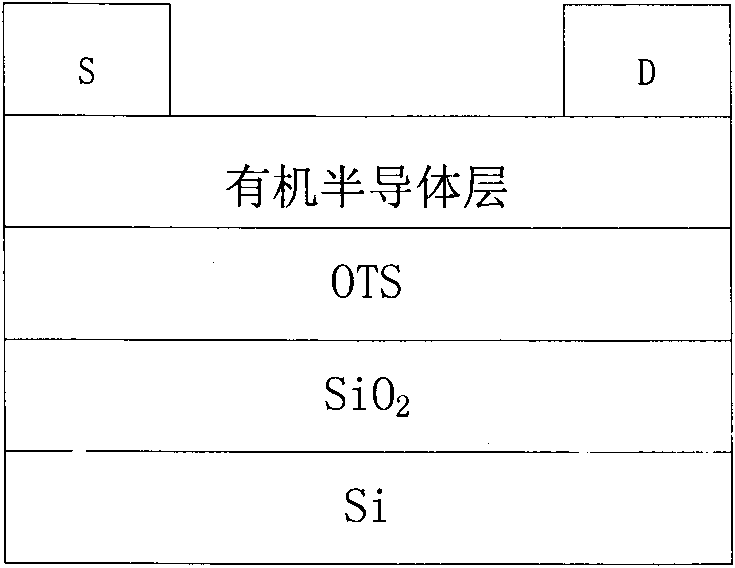
Naphthalene tetracarboxylic acid diimide-dithiophene quinoxaline copolymer, its preparation method and applications
A technology of naphthalene tetracarboxylic acid diimide and quinoxaline copolymer, which is applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, luminescent materials, etc., can solve the problem of reducing the photoelectric conversion efficiency of organic solar cells and cannot effectively Utilize the problems of sunlight and insufficient emission spectrum matching to achieve the effects of increasing mobility, improving solution processability, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Poly N,N'-bis-(2-octyldecyl)-1,4,5,8-naphthalene diimide-(2-methyl-3-methoxy)dithieno[3,2 -f: Synthesis of 2',3'-h]quinoxaline (n=30):
[0035]
[0036] Under the protection of nitrogen, to the compound N,N'-bis-(2-octyldecyl)-2,6-dibromo-1,4,5,8-naphthalene diimide 0.5mmol, 6,9 - A solution of 0.5 mmol of bistributyltin-(2-methyl-3-methoxy)dithieno[3,2-f:2',3'-h]quinoxaline in DMF (18 mL). Bubble 0.5h to remove residual oxygen. Then join Pd 2 (dba) 3 (0.0.14g, 0.015mol) and P(o-Tol) 3 (0.0083g, 0.027mmol) and bubbling for 0.5h to remove residual oxygen, then heated to 80°C for 48 hours. The mixture was added dropwise to methanol for settling. Suction filtration, washing with methanol, and drying. It was then dissolved in toluene, added to an aqueous solution of sodium diethyldithiocarbamate, and the mixture was heated to 90°C and stirred overnight. The organic phase was subjected to column chromatography on alumina, eluting with chlorobenzene. The organic so...
Embodiment 2
[0038] Poly N,N'-bis-(2-hexyloctyl)-1,4,5,8-naphthalene diimide-(2-hexyl-3-decyl)dithieno[3,2-f: Synthesis of 2',3'-h]quinoxaline (n=16):
[0039]
[0040] Under the protection of nitrogen, to the compound N, N'-bis-(2-hexyloctyl)-2,6-dibromo-1,4,5,8-naphthalene diimide 0.5mmol, 6,9- A solution of 0.5 mmol of bistributyltin-(2-hexyl-3-decyl)dithieno[3,2-f:2',3'-h]quinoxaline in dioxane (15 mL). Bubble 0.5h to remove residual oxygen. Then add Pd(PPh 3 ) 2 Cl 2 10mg, bubbled for 0.5h to remove residual oxygen, and then heated to 120°C for 24 hours. The mixture was added dropwise to methanol for settling. Suction filtration, washing with methanol, and drying. It was then dissolved in toluene, added to an aqueous solution of sodium diethyldithiocarbamate, and the mixture was heated to 90°C and stirred overnight. The organic phase was subjected to column chromatography on alumina, eluting with chlorobenzene. The organic solvent was removed under reduced pressure and met...
Embodiment 3
[0042] Poly N,N'-bis-(2-methyltetradecyl)-1,4,5,8-naphthalene diimide-(2,3-di-eicosyl)dithieno[3 , 2-f: Synthesis of 2',3'-h]quinoxaline (n=25):
[0043]
[0044] Under the protection of nitrogen, to the compound N,N'-bis-(2-methyltetradecyl)-2,6-dibromo-1,4,5,8-naphthalene diimide 0.5mmol, 6 , 9-bistributyltin-(2,3-di-eicosyl)dithieno[3,2-f:2',3'-h]quinoxaline 0.5mmol solution in toluene / THF (30mL) . Bubble 0.5h to remove residual oxygen. Then add Pd(PPh 3 ) 4 8mg, bubbled for 0.5h to remove residual oxygen, and then heated to 50°C for 72 hours. The mixture was added dropwise to methanol for settling. Suction filtration, washing with methanol, and drying. Then it was dissolved in toluene, added to the aqueous solution of sodium diethyldithiocarbamate, and then the mixture was heated to 80C and stirred overnight. The organic phase was subjected to column chromatography on alumina, eluting with chlorobenzene. The organic solvent was removed under reduced pressure an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com