Injectable temperature-sensitive hydrogel and preparation method thereof
A temperature-sensitive hydrogel and room temperature technology, applied in the field of biomedical polymer materials, can solve problems such as easy clogging of needles, and achieve the effects of easy injection, small hydrodynamic volume, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
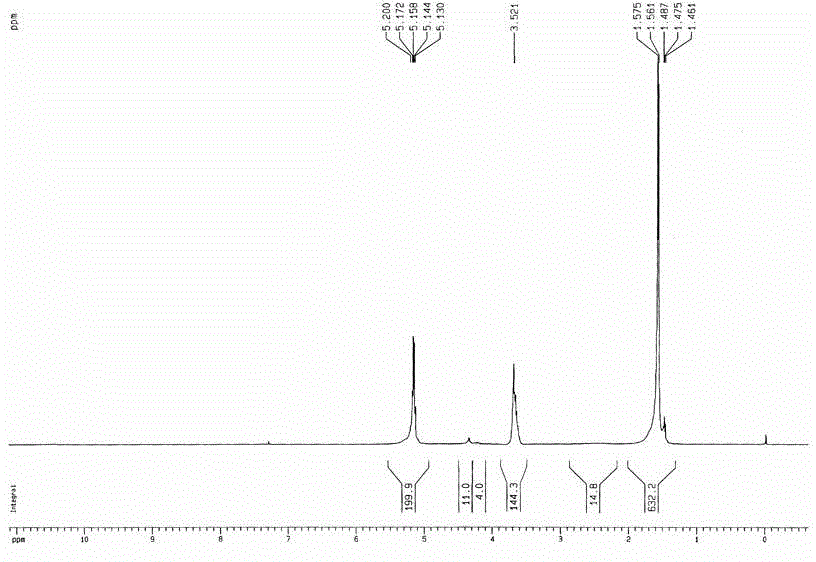
[0030] Weigh 20g of L-lactide and 0.945g of pentaerythritol into a round bottom flask, add 0.03% stannous octoate, and react at 160°C for 8 hours under normal pressure; after the reaction, add 40ml of DMSO to dissolve it, then add 4.17g of succinic anhydride, 4ml of triethylamine and 0.5g of DMAP were reacted at room temperature, and then the product was added into water until the precipitation was complete to obtain light yellow solid PET-PLLA-SA with a viscosity average molecular weight of 2270. Weigh a certain amount of PET-PLLA-SA and mPEG 350 Dissolve in chloroform, add DMAP and DCC, react overnight at room temperature, dissolve the product in water, heat up until a precipitate forms, filter to obtain a four-arm PLLA-mPEG block copolymer with a viscosity-average molecular weight of 3760; from HNMR It can be seen from the spectrum ( figure 1 ), the original two peaks of pentaerythritol, one is 4.688 (CC H 2 OH), 3.488 (CCH 2 o H ), after polymerization, peak 3.488 has...
Embodiment 2
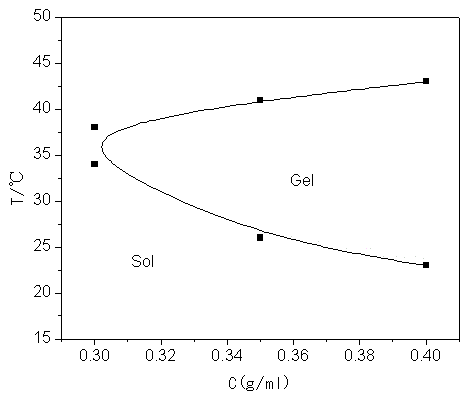
[0032] Weigh 20g of L-lactide and 0.945g of pentaerythritol into a round bottom flask, add 0.03% zinc lactate, replace with N2 gas, and react at 120°C for 8 hours under normal pressure; after the reaction, add 40ml of DMSO to make it Dissolve, then add 4.17g succinic anhydride, 4ml triethylamine and 0.5g DMAP, and react at 25°C; then add the reactant to water to obtain light yellow solid PET-PLLA-SA with a viscosity average molecular weight of 2340. Weigh a certain amount of PET-PLLA-SA and mPEG 350 Dissolve in dichloromethane, add DMAP and DCC, react overnight at room temperature, dissolve the product in water, heat up until a precipitate forms, filter to obtain a four-armed PLLA-mPEG block copolymer with a viscosity-average molecular weight of 3660; PLLA-mPEG was added to water under low conditions to obtain an aqueous copolymer solution, which gelled rapidly at a temperature of 37°C.
Embodiment 3
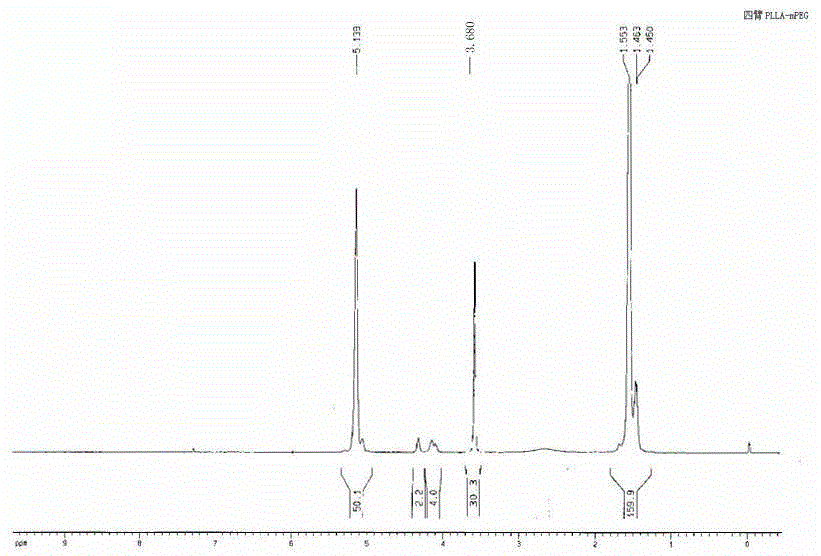
[0034] Weigh 20g of L-lactide and 0.9615g of glycerol in a round-bottomed flask, add 0.03% stannous octoate, replace with N2 gas, and react at 160°C for 8 hours; after the reaction, add 40ml of DMSO to make After it was dissolved, 5.208g of succinic anhydride, 4ml of triethylamine and 0.5g of DMAP were added and reacted at 25°C; then the reactant was added into water to obtain a light yellow solid with a viscosity-average molecular weight of 2770. Weigh a certain amount of light yellow solid and mPEG 500 Dissolve in dichloromethane, add DMAP and DCC, react overnight at room temperature, dissolve the product in water, heat up until a precipitate forms, filter, and obtain a three-arm PLLA-mPEG block copolymer with a viscosity-average molecular weight of 4310; It can be seen from the HNMR spectrum ( figure 2 ), glycerol and L-lactide polymerization, HNMR peak area analysis, 4.38 [OCO(C H )OH] is 11, 4.25 (C H ) is 4, the ratio of the two is about 3, indicating that the polyme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com