Method for preparing piceatannol
A technology of piceatannol and potassium tert-butoxide, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve problems such as low yield of piceatannol, and achieves safety and high purity of reactants. , easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
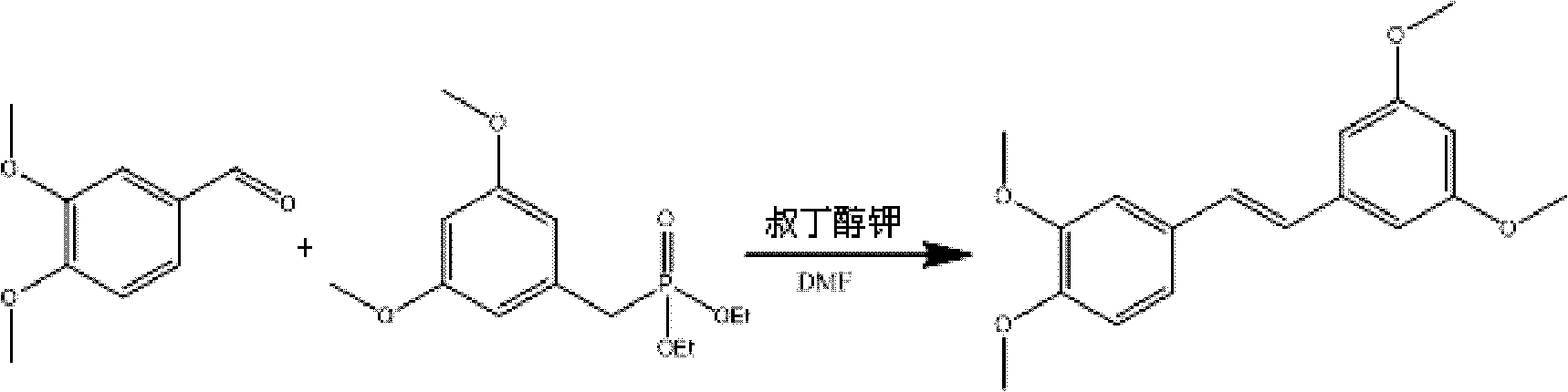
[0030] Embodiment 1: 3,4,3',5'-Tetramethoxystilbene yield comparative test in different catalysts and solvents
[0031]Dissolve 3,5-dimethoxybenzyl diethyl phosphate in different solvents, under the protection of nitrogen, drop different catalysts, keep different temperatures, stir for 1 hour, and then add 3,5-dimethoxybenzene After formaldehyde was dissolved in different solvents, it was added dropwise to the reaction system, and after the dropwise addition was completed, it was stirred at room temperature for 1 hour. After the reaction was completed, add ethyl acetate for extraction, wash with water, collect the ethyl acetate portion, and recover the solvent under reduced pressure to obtain a crude product, dissolve it with ethyl acetate and petroleum ether, filter, collect the filter cake, and dry. The solvent was recovered from the filtrate to dryness under reduced pressure, and the residue was subjected to silica gel column chromatography to finally obtain a white solid, ...
Embodiment 2
[0035] Embodiment 2: 3,4,3', the preparation of 5'-tetramethoxystilbene
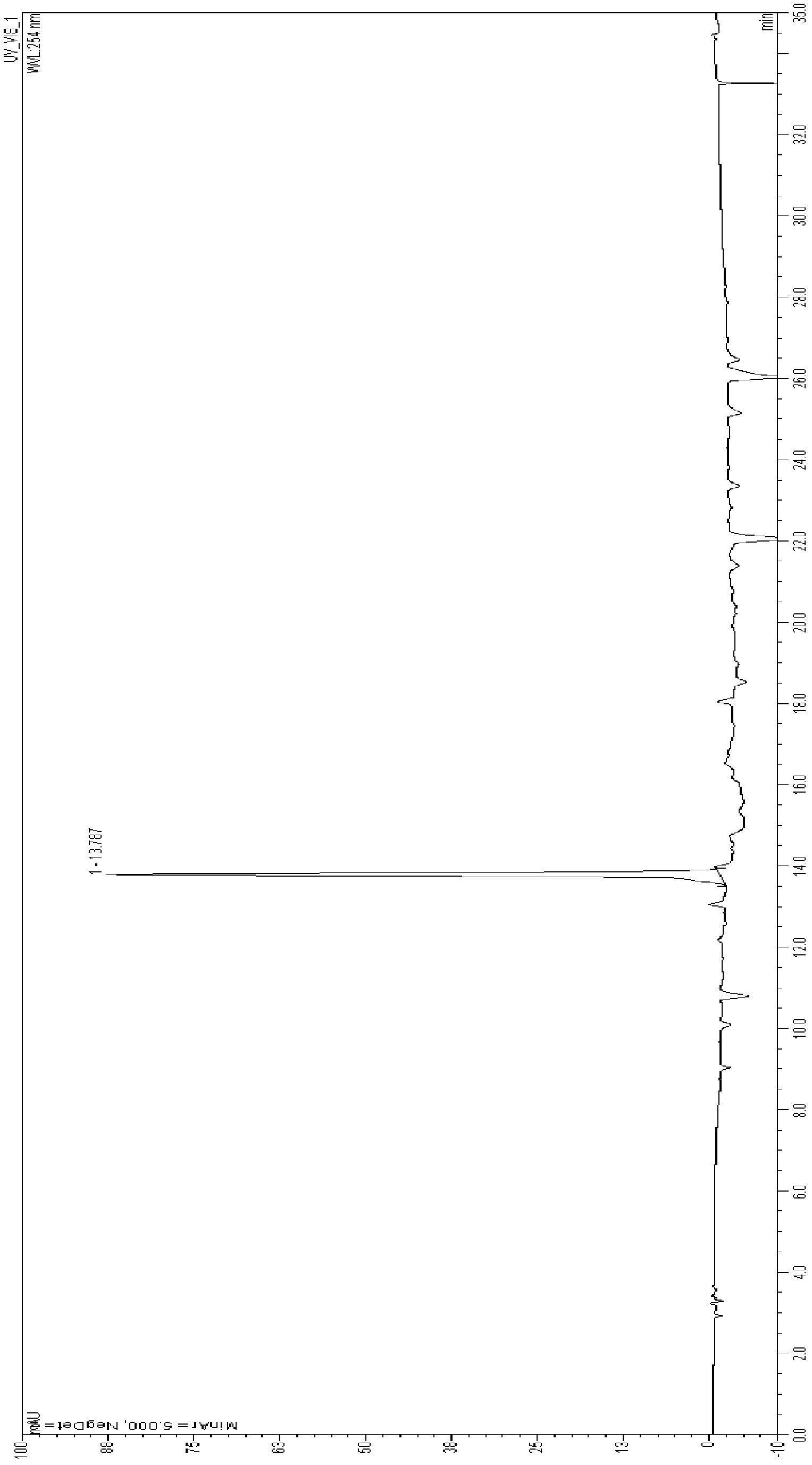
[0036] Under nitrogen protection, 245 g (0.85 mol) of 3,5-dimethoxybenzyl diethyl phosphate was added to 2.5 L dimethylformamide solution, and 95.4 g (0.85 mol) of potassium tert-butyl alkoxide was added in portions. mol), control the temperature at 0°C, stir for 1 hour, then add 120 g (0.72 mol) of 3,5-dimethoxybenzaldehyde (CASNo: 120-14-9) dropwise, and stir at room temperature for 1 Hour. The progress of the reaction was monitored by TLC (petroleum ether V:ethyl acetate V=1:4). When the reaction was completed, keep stirring, and slowly add 4L of ice water, resulting in a large amount of precipitation. Ethyl acetate was added for extraction, the ethyl acetate layer was washed 3 times with water, and dried over anhydrous sodium sulfate. The organic layer was concentrated to dryness under reduced pressure to obtain a crude product as a pale yellow solid. The solid crude product was washed with petro...
Embodiment 3
[0037] Embodiment 3: the preparation of picatanol
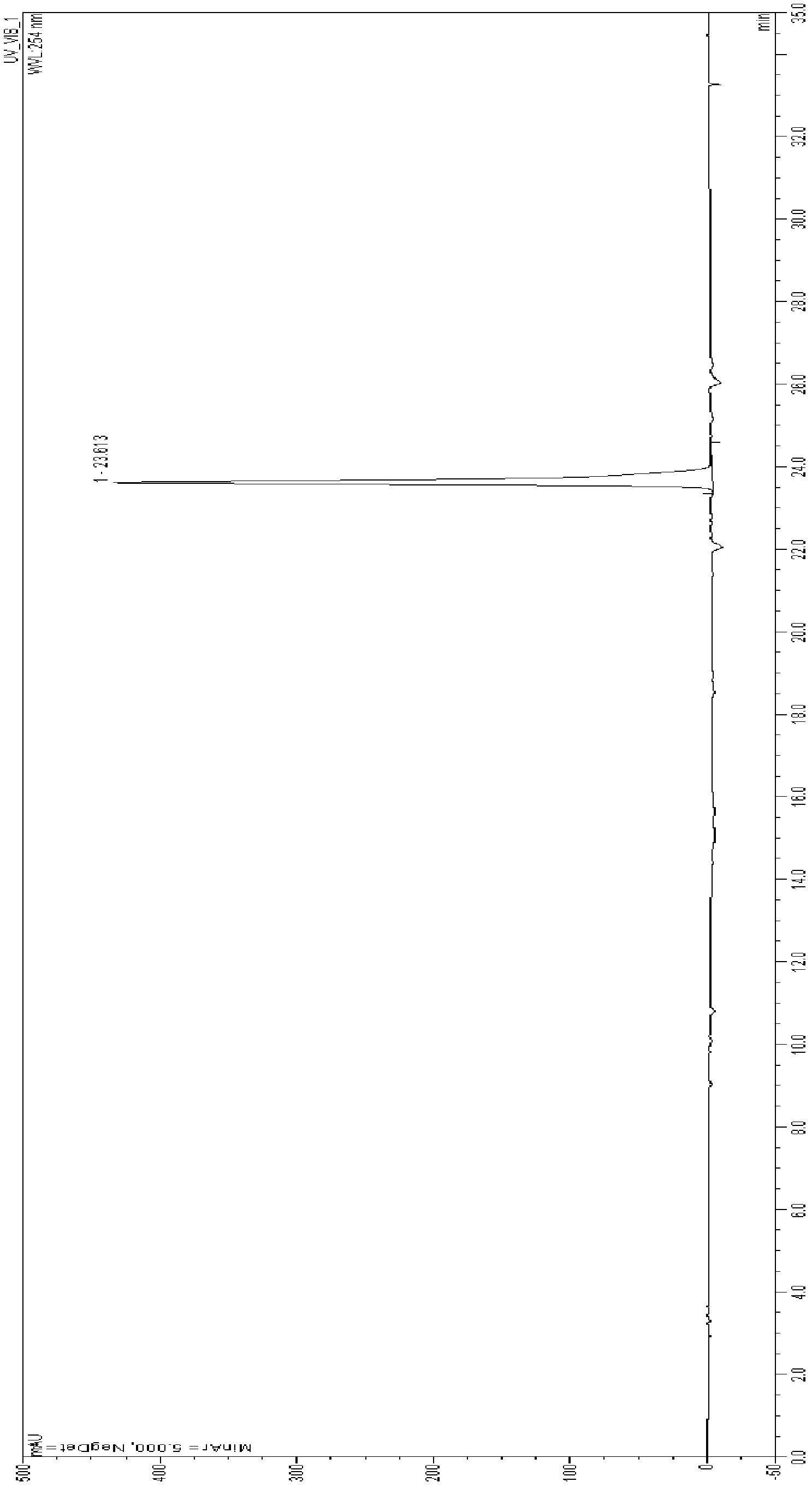
[0038] Under nitrogen protection, 120.4 g (0.4 mol) of 3,4,3′,5′-tetramethoxystilbene was dissolved in anhydrous dichloromethane solution, and 601.4 g (0.85 mol) of boron tribromide was added dropwise. mol) of anhydrous dichloromethane solution, keep the temperature constant at -30°C, after the dropwise addition, stir at room temperature for 2 hours, and monitor the reaction progress by HPLC. When the reaction reached the end point, the stirring was continued, and 4L of ice water was slowly added, and a large amount of precipitation was precipitated. Suction filtration, wash the filter cake with water. The filter cake was dissolved in ethyl acetate and washed with water. The organic layer was concentrated to dryness under reduced pressure to obtain a pink solid crude product, which was stirred with ethyl acetate and petroleum ether, and a large amount of precipitation was precipitated, filtered, and the filter cake was wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com