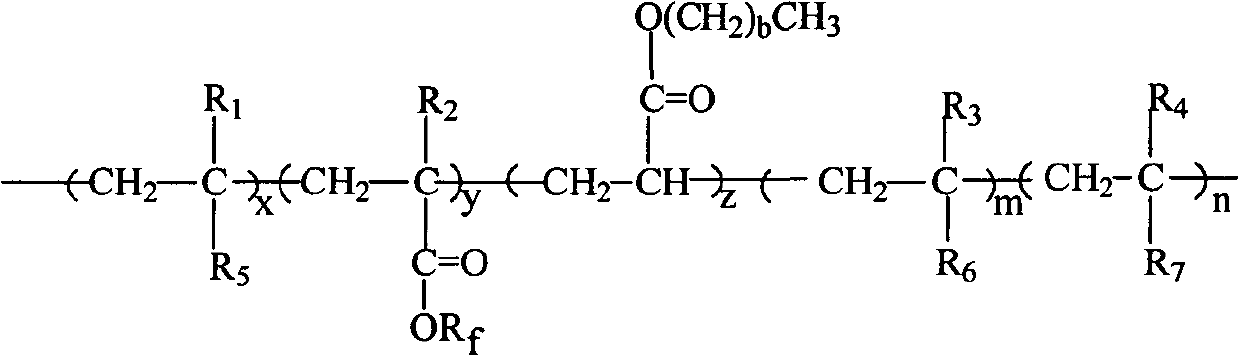
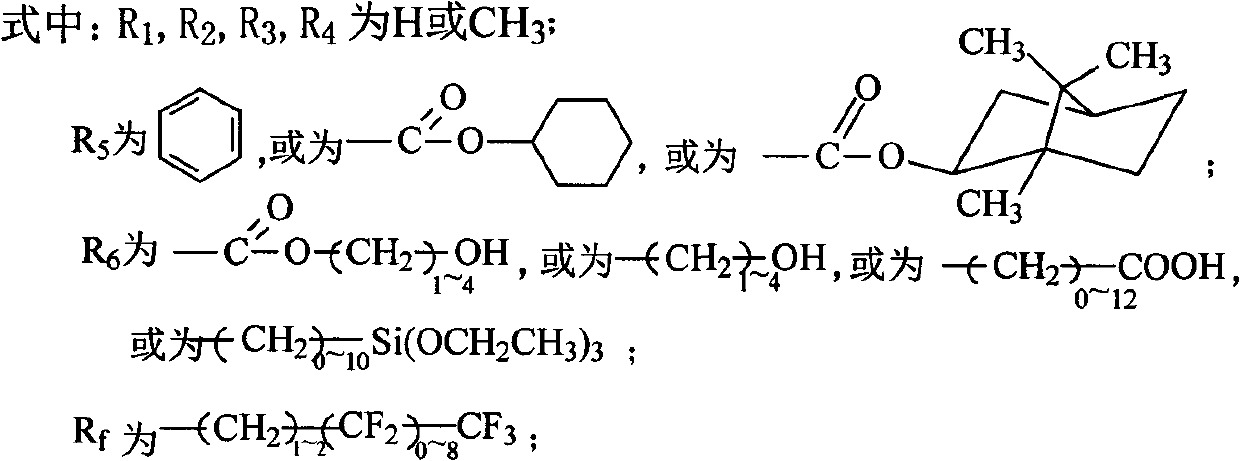
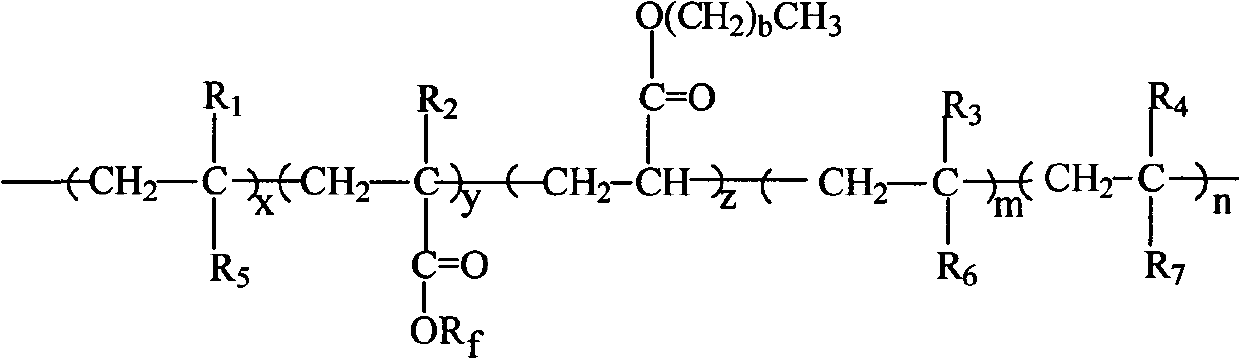
Fluorine silicon acrylate copolymer, preparation process thereof and application thereof
A technology of fluorosilicone acrylate and acrylate, which is applied in the field of fluorosilicone acrylate copolymer and its preparation, can solve the problem that the strength and hardness of fluorosilicone acrylate copolymer are not ideal, and reports have not yet been found to limit fluorosilicone acrylate copolymer. Application and other issues, to achieve the effect of simple synthesis method, strong hydrophobicity, good anti-ice and snow performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In reactor (250ml), add 10g methyl isobutyl ketone and 0.05g azobisisobutyronitrile, blow nitrogen, be heated to temperature 60 ℃, drop two kinds of mixed solutions to reactor again, wherein a kind of mixed solution is made of 12g cyclohexyl methacrylate, 9g octyl acrylate, 2g hydroxyethyl methacrylate and 15g methyl isobutyl ketone, another mixed solution consists of 5g methyl isobutyl ketone and 0.05g azobisisobutyronitrile composition, reacted for 3h, then slowly added dropwise the remaining 0.05g of azobisisobutyronitrile, 3g of trifluorooctyl methacrylate and 3g of trifluoroethyl acrylate, 1g of 4-methacryloxybutyl-7 Styryl POSS and the remaining 5 g of methyl isobutyl ketone were reacted for 24 hours, and the temperature was lowered to obtain a translucent emulsified solution.
[0025] The number average molecular weight of the copolymer was measured by GPC-MwA gel chromatography 16000, molecular weight distribution index The contact angle of the copolymer wit...
Embodiment 2
[0027] Add 10 g of xylene and 0.2 g of azobisisobutyronitrile into a 250 mL reactor, blow nitrogen, and heat to 80°C. Then add dropwise a mixed solution consisting of 12g cyclohexyl acrylate, 6g butyl acrylate, 0.72g vinyltriethoxysilane and 5g xylene, and dropwise add a solution consisting of 5g xylene and 0.2g azobisisobutyronitrile The solution. After reacting for 3 hours, 0.2 g of azobisisobutyronitrile, 2.4 g of dodecafluoroheptyl methacrylate and 5 g of xylene were added dropwise. After continuing to react for 24 h, the temperature was lowered to room temperature, and a translucent milky solution was obtained.
[0028] Using GPC-MwA gel chromatograph, the GPC method measures the number average molecular weight of the copolymer molecular weight distribution index The contact angle of the coating film with water was 110°.
Embodiment 3
[0030] Add 30 g of butyl acetate into the reactor (250 ml), blow nitrogen, and heat to 80° C. A solution composed of 12g cyclohexyl methacrylate, 15.6g octadecyl methacrylate, 2.4g undecylenic acid, and 15g methyl isobutyl ketone was added dropwise, while another constant pressure funnel was added dropwise from 15g A solution composed of butyl acetate and 0.3g of dibenzoyl peroxide was heated to 110°C and reacted for about 3 hours. Then the remaining 0.15g of dibenzoyl peroxide, 6g of perfluoroalkylethyl acrylate, and 15g of butyl acetate were slowly added dropwise. After 24 hours of reaction, the temperature was lowered to obtain a translucent emulsified solution. The above-mentioned 10g of fluorosilicone acrylate copolymer was dissolved in a mixture of 65g of ethyl acetate and 25g of toluene at room temperature to prepare a solution, ultrasonically oscillated, coated on a clean glass sheet after dissolution, and dried at room temperature for 24 hours.
[0031] Copolymer num...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com