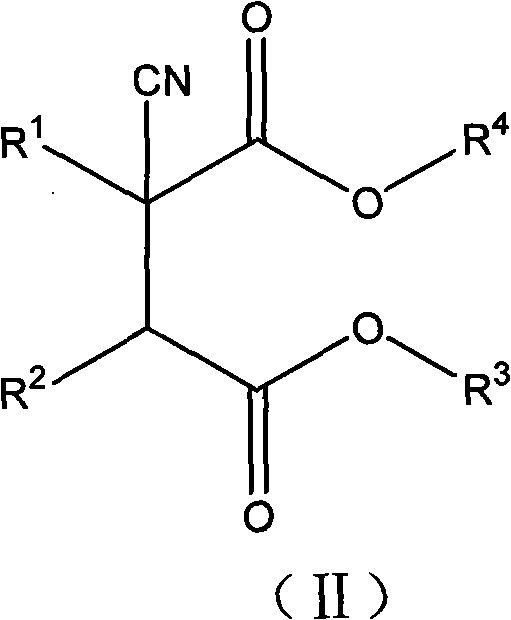
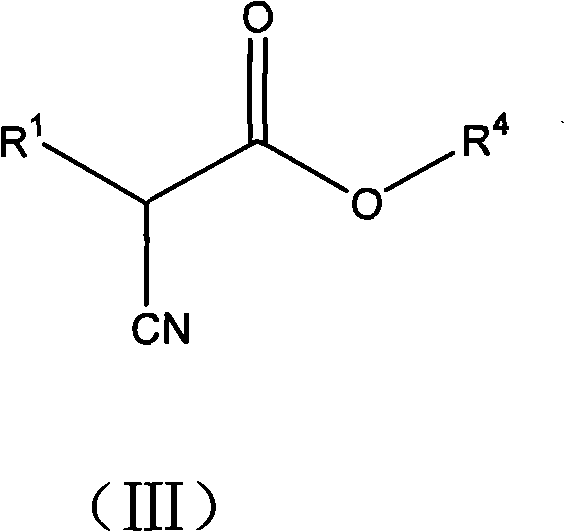
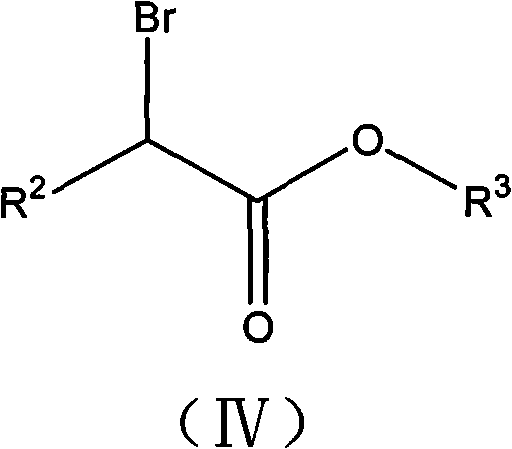
2-cycloalkyl-3-secondary alkyl-2-cyansuccinate compounds, preparation method thereof and application thereof
A cyanosuccinate and compound technology, which is applied in the fields of organic synthesis and olefin polymerization, can solve the problems of narrow relative molecular mass distribution of polymers, poor polymer regularity, low catalytic activity, etc., and achieves easy industrial preparation and production. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Preparation of Diethyl 2-cyclopentyl-3-isopropyl-2-cyanosuccinate
[0085] Under nitrogen protection, the prepared solution of 4.07g potassium ethylate dissolved in 15% ethanol was added dropwise to a 100ml flask containing 8g ethyl 2-cyclopentyl-2-cyanoacetate. The reaction was stirred at room temperature, and then the solvent was removed to obtain a white solid, which was dissolved in 150ml of purified tetrahydrofuran, and then slowly added dropwise to the tetrahydrofuran solution in which 9.18g of ethyl 2-bromoisovalerate was dissolved. Under the reaction 8h. The solvent was then removed by evaporation to give a mixture of a red viscous liquid and a white solid. Extract with ether, wash with water, 5% sodium bicarbonate, then water until the organic phase is neutral. The organic phase was dried with anhydrous magnesium sulfate for 12 h. Filter and remove the solvent to obtain 10.2 g of light yellow transparent liquid. It was confirmed by high-resolution electrosp...
Embodiment 2
[0092] Preparation of Diethyl 2-cyclopentyl-3-isopropyl-2-cyanosuccinate
[0093] Repeat Example 1, but replace tetrahydrofuran solvent with acetonitrile, obtain compound 2-cyclopentyl-3-isopropyl-2-cyanodiethyl succinate, the product is separated with a chromatographic column filled with silica gel (elution Liquid: petroleum ether and chloroform) to obtain light yellow transparent liquid (yield: 67.7%). The mass spectrum and infrared analysis spectrum of the product are consistent with the structure of the target compound.
Embodiment 3
[0095] Preparation of Diethyl 2-cyclohexyl-3-isopropyl-2-cyanosuccinate
[0096] Under nitrogen protection, the prepared 3.32 g of potassium ethylate was added dropwise into a 100 ml flask containing 7 g of ethyl 2-cyclohexyl-2-cyanoacetate. The reaction was stirred at room temperature, and then the solvent was removed to obtain a white solid, which was dissolved in 150ml of purified tetrahydrofuran, and then slowly added dropwise to 30ml of tetrahydrofuran solution in which 7.46g of ethyl 2-bromoisovalerate was dissolved. Under the condition of reaction 8h.
[0097] After the reaction was completed, the solvent was removed first to obtain a mixture of red viscous liquid and white solid. Extract with ether, wash with water, 5% sodium bicarbonate, then water until the organic phase is neutral. Dry the organic phase with anhydrous magnesium sulfate for 12 h, filter, and remove the solvent to obtain 8.9 g of a yellow transparent liquid. The generation of the target compound wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com