Recombinant protein A efficiently combined with IgG (Immunoglobulin G) and construction method of engineering bacterium thereof
A technology of recombinant protein and engineering bacteria, which is applied in the fields of molecular biology and genetic engineering, can solve the problems of increased risk of protein A isolation, poor extraction efficiency of protein A, and difficulty in large-scale extraction, so as to increase production efficiency and work Safety, simple extraction method, and the effect of increasing extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Design of primers for recombinant protein A antibody binding region
[0020] According to the gene sequence published by NCBI, the primer P was designed using DNAMAN software. 1 and P 2 .
[0021] P 1 : 5'-ACCGCCATGGTATTGAAAAAGAAAAACATT-3'Noc I
[0022] P 2 : 5'-ACCGGGATCCTTAACATAGTTCGCGACGACGTCC-3'Bam H I
[0023] The part in italics in the primer is the restriction site, in the primer P 2 A cysteine codon and a stop codon were added. The purpose of designing primers based on the IgG antibody binding region of protein A is to effectively reduce the length of the peptide chain and reduce the steric hindrance effect when binding the medium and IgG. A cysteine is designed at the C-terminus of protein A, the purpose is to combine the activation medium with the -SH of cysteine, and improve the adsorption capacity for IgG.
Embodiment 2
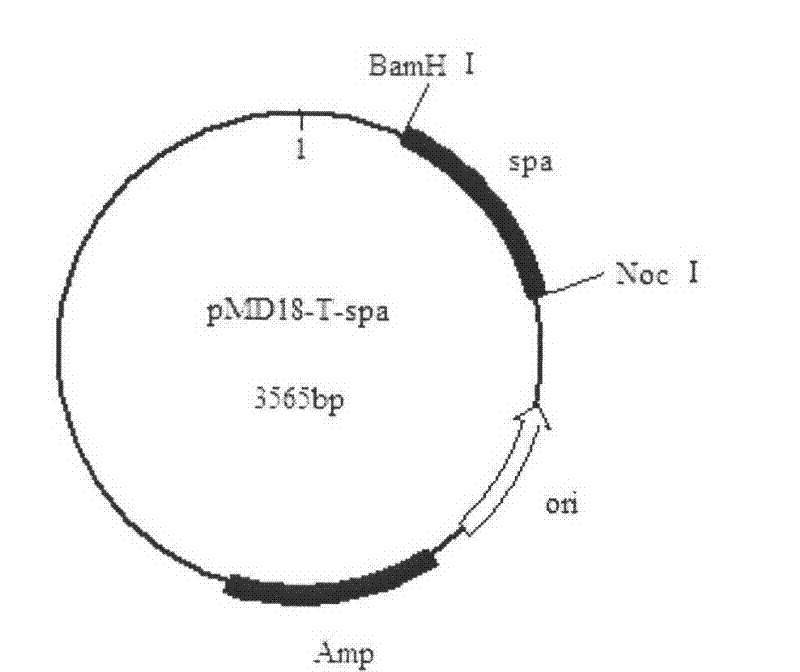
[0024] Embodiment 2: Construction of recombinant plasmid pMD18-T-spa
[0025] [1] Chromosomal DNA extracted from Staphylococcus aureus as a template, extraction method: pick a single colony from a fresh Staphylococcus aureus plate with an inoculation loop and inoculate it in LB liquid medium, shake overnight at 37°C, and take out the next day Centrifuge 400μl of the bacterial solution at 8000r / min for 3min, discard the supernatant, wash the cells once with 0.5ml TE, discard the supernatant by centrifugation, suspend the cells sufficiently with 0.5ml TE, add 50μl of 10mg / ml lysozyme solution, mix well, 37 ℃ water bath for 2-3 hours, add 100μl 10% SDS, mix well, keep warm at 37℃ for 30min, add 50μl 10mg / ml proteinase K, react at 65℃ for 3h, mix once every half hour during this period, add an equal volume of saturated phenol, mix Evenly, centrifuge at 8000r / min for 5min, transfer the supernatant to another centrifuge tube, add an equal volume of saturated phenol to the supernatan...
Embodiment 3
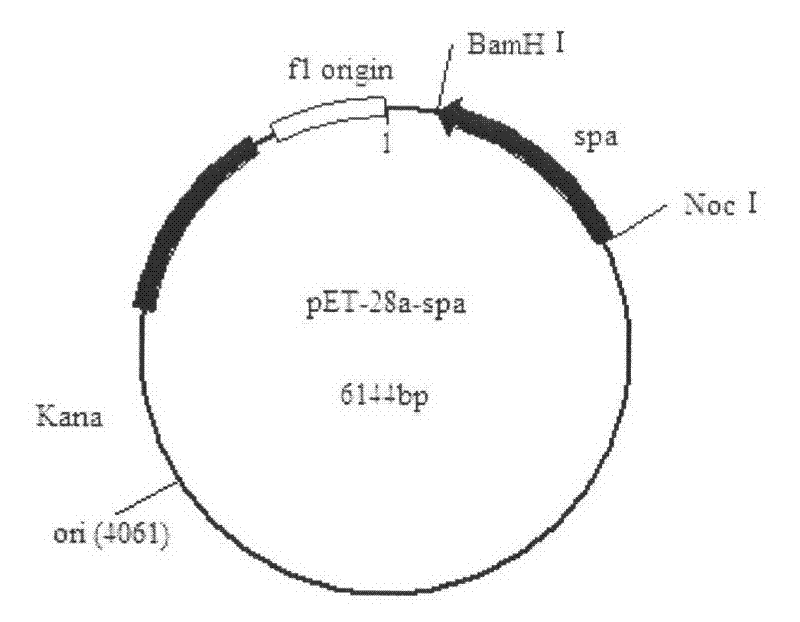
[0028] Embodiment 3: Construction of recombinant plasmid pET-28a-spa
[0029] Construct the recombinant vector pET-28a-spa, double digest the plasmid extracted in Example 2 [3] and plasmid pET-28a with BamH I and Noc I, use the gel recovery box to recover and connect, connection system: target gene Digested product 7.5 μl, pET-28a digested product 0.5 μl, T4 DNA ligase buffer 1 μl, T4 DNA ligase 1 μl, ligated overnight at 16°C. The ligated recombinant plasmid pET-28a-spa was transformed into competent E.coil BL21, the transformation method was referred to Example 2[3], and positive colonies were picked with a Kanamycin plate (Kana+LB). The plasmid was extracted after culturing overnight on a shaker at 37°C, added glycerol after enzyme digestion was verified to be correct, and stored in a -20°C refrigerator for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com