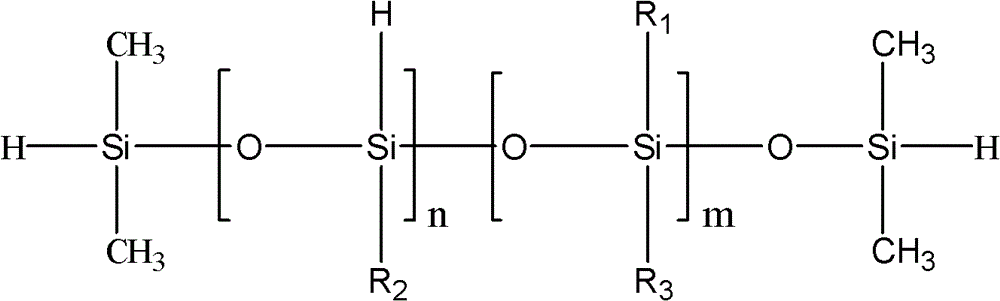
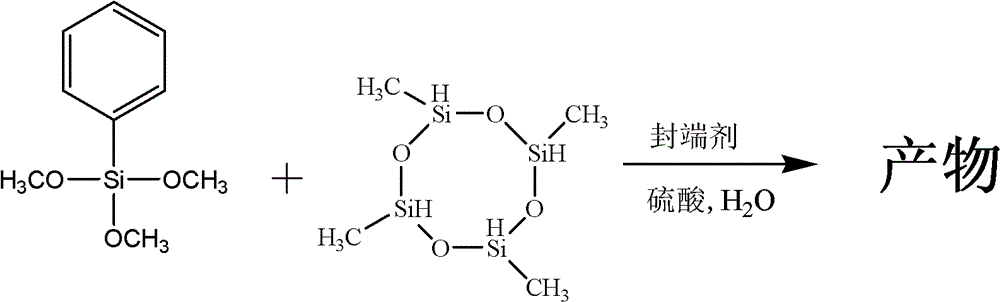
Preparation method for phenyl hydrogen-containing silicone oil
A technology of phenyl hydrogen-containing silicone oil and phenyl alkoxysilane, applied in chemical instruments and methods, adhesive additives, polymer adhesive additives, etc. Poor controllability, poor product stability and other problems, to achieve the effect of easy control, simple operation and wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of the present embodiment phenyl hydrogen-containing silicone oil comprises the following steps successively:
[0026] (1) Use phenylalkoxysilane and cyclosiloxane as basic reaction monomers, add organic solvent, water and end-capping agent, and carry out hydrolysis polycondensation reaction under the action of acidic catalyst;
[0027] The above-mentioned phenylalkoxysilane is phenyltrimethoxysilane, and its amount is 14.50 g (0.0731 mol). The above-mentioned cyclosiloxane is tetramethyltetrahydrocyclotetrasiloxane, and its usage amount is 10.67 g (0.0443 mol). In phenylalkoxysilane and cyclosiloxane, the ratio of the total moles of phenyl groups to the total moles of Si—H groups is about 0.413:1.
[0028] Above-mentioned organic solvent is toluene, and its consumption is 40.00g. The consumption of organic solvent is about 1.59 times of the total mass of basic reaction monomers.
[0029] The above-mentioned water is deionized water, and its co...
Embodiment 2
[0039] The preparation method of the present embodiment phenyl hydrogen-containing silicone oil comprises the following steps successively:
[0040] (1) Use phenylalkoxysilane and cyclosiloxane as basic reaction monomers, add organic solvent, water and end-capping agent, and carry out hydrolysis polycondensation reaction under the action of acidic catalyst;
[0041] In this step (1), the hydrolysis polycondensation reaction is carried out under stirring, the reaction time of the hydrolysis polycondensation reaction is 10 hours, and the reaction temperature is 100°C.
[0042] The above-mentioned phenylalkoxysilane is phenyltrimethoxysilane, and its amount is 19.80 g (0.0999 mol). The above-mentioned cyclosiloxane is tetramethyltetrahydrocyclotetrasiloxane, and its dosage is 2.60 g (0.0108 mol). In phenylalkoxysilane and cyclosiloxane, the ratio of the total moles of phenyl groups to the total moles of Si—H groups is 2.3125:1.
Embodiment 3
[0052] The preparation method of the present embodiment phenyl hydrogen-containing silicone oil comprises the following steps successively:
[0053] (1) Use phenylalkoxysilane and cyclosiloxane as basic reaction monomers, add organic solvent, water and end-capping agent, and carry out hydrolysis polycondensation reaction under the action of acidic catalyst;
[0054] In this step (1), the hydrolysis polycondensation reaction is carried out under stirring, the reaction time of the hydrolysis polycondensation reaction is 14 hours, and the reaction temperature is 20°C.
[0055] The above-mentioned phenylalkoxysilane is phenyltrimethoxysilane, and its amount is 19.80 g (0.0999 mol). The above-mentioned cyclosiloxane is tetramethyltetrahydrocyclotetrasiloxane, and its usage amount is 4.16 g (0.0173 mol). In phenylalkoxysilane and cyclosiloxane, the ratio of the total moles of phenyl groups to the total moles of Si—H groups is about 1.44:1.
[0056] Above-mentioned organic solvent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com