Super-amphiphobic polymer and super-amphiphobic surface prepared from super-amphiphobic polymer
A super-amphiphobic, polymer technology, applied in the addition of inorganic compounds, plant fibers, water repellents, etc., can solve the problems of difficult industrial production and application, insufficient bonding strength, easy surface damage, etc., and achieve good surface hydrophobicity. and oleophobicity, simple preparation method and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A preparation method of super amphiphobic polymer, comprising the following steps:
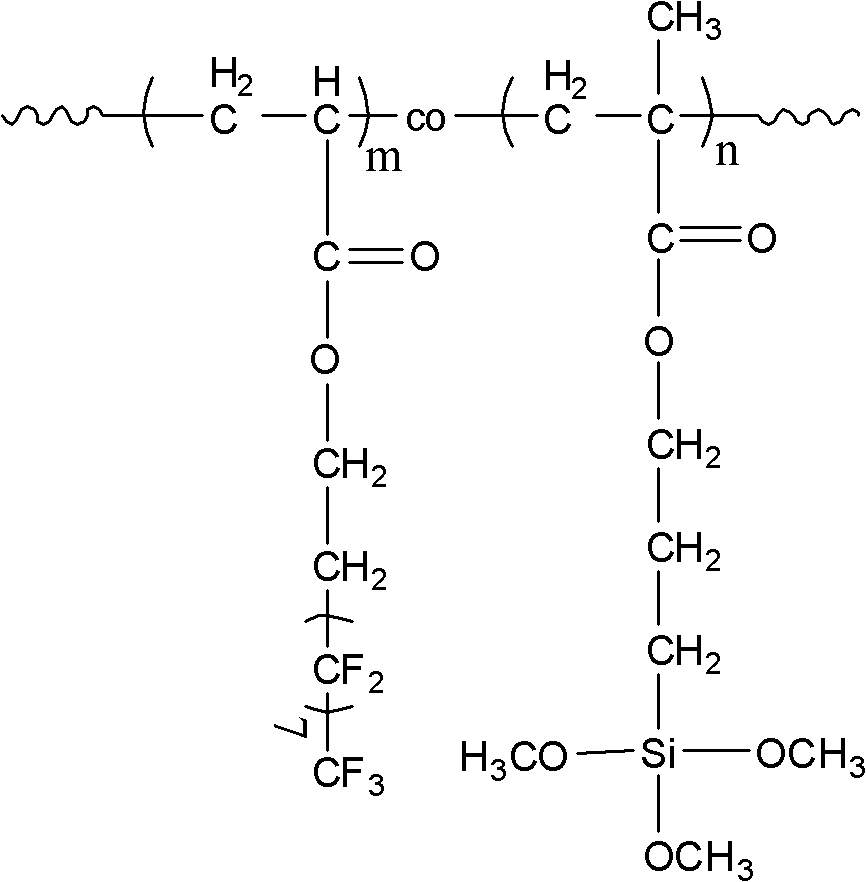
[0053] Add 15g of perfluorooctyl ethyl acrylate, 1.852g of methacryloxypropyltrimethoxysilane, 0.203g of 2-bromoisobutyric acid monomethoxyethylene glycol, 0.737g in a 100ml round bottom flask g 4,4'-dinonyl-2,2'-bipyridine and 4ml benzotrifluoride, the reaction system was stirred and dissolved, and the argon gas was bubbled for 30min, and then the oxygen was removed, and then the reaction system was transferred to a container equipped with 0.1294g In a 100ml round bottom flask of cuprous bromide, carry out the polymerization reaction at 90°C for 8h, the reaction product is precipitated in methanol, washed with methanol and then washed with n-hexane, and then vacuum-dried at 40°C for 24h to constant weight to obtain the product .
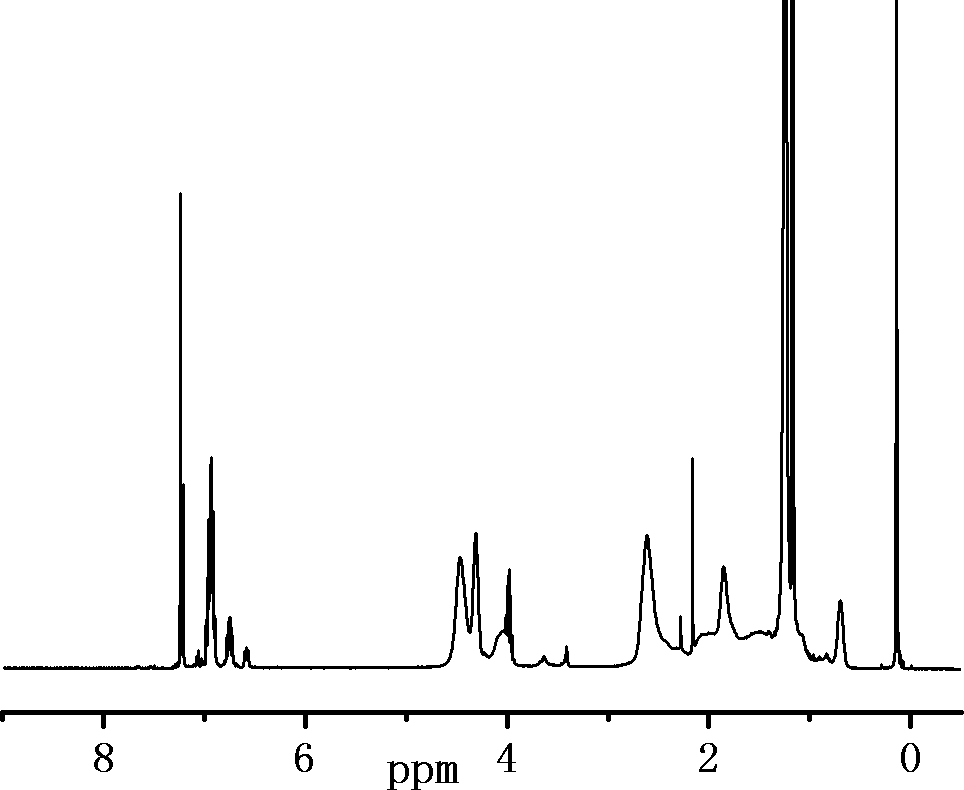
[0054] The spectral analysis of the product is as follows: 1 H-NMR (hexafluorobenzene / CDCl 3 = 2 / 1 volume ratio): 2.60 (m, -CH 2 -(CF 2 ) 7 CF 3 , 2H)...
Embodiment 2
[0058] A preparation method of super amphiphobic polymer, comprising the following steps:
[0059] In a 100ml round bottom flask, add 15g perfluorooctyl ethyl acrylate, 1.852g methacryloxypropyltrimethoxysilane, 0.174g AIBN as initiator and 50ml trifluorotoluene, and stir the reaction system Dissolved, bubbled with argon for 30 minutes, and polymerized at 90°C for 8 hours. The reaction product was precipitated in methanol, washed with methanol and then washed with n-hexane, and then vacuum-dried at 40°C for 24 hours to constant weight to obtain the product.
[0060] The spectral analysis of the product is as follows: 1 H-NMR (hexafluorobenzene / CDCl 3 = 2 / 1 volume ratio): 2.60 (m, -CH 2 -(CF 2 ) 7 CF 3 , 2H), 3.42(m, -O-CH 3 , 3H), 0.65(m, -CH 2 -Si(OCH 3 ) 3 , 2H); Specific NMR chemical shift and figure 1 Similarly, it can be deduced that the structure of the product of this example is the same as formula III in combination with the NMR image. It can be inferred that...
Embodiment 3
[0062] Preparation of polymer nanospheres containing hydroxyl groups on the surface:
[0063] Under stirring, in the there-necked flask of 500 milliliters, gradually add the mixture of 130 milliliters of distilled waters, 4.80 grams (48.0 millimoles) methyl methacrylate and 0.4 grams (2.0 millimoles) ethylene glycol dimethacrylate, and 41 mg (0.15 micromol) of potassium peroxodisulfate in water (5 mL). Nitrogen was blown through the reaction system at 25°C for 15 minutes to remove oxygen in the system. Then heated to 90° C. in an oil bath, and reacted for 2 hours.
[0064] 43 milliliters of the solution was taken out from the above system, added to a 250 milliliter three-necked flask filled with nitrogen, and 0.5 milliliters of a solution of 2.4 milligrams (14.6 micromoles) of azobisisobutyronitrile in tetrahydrofuran was added. After stirring for 15 minutes at 25°C, it was heated to 90°C. Then slowly add the solution containing 0.4 g (1.9 mmol) ethylene glycol diester 2-ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com